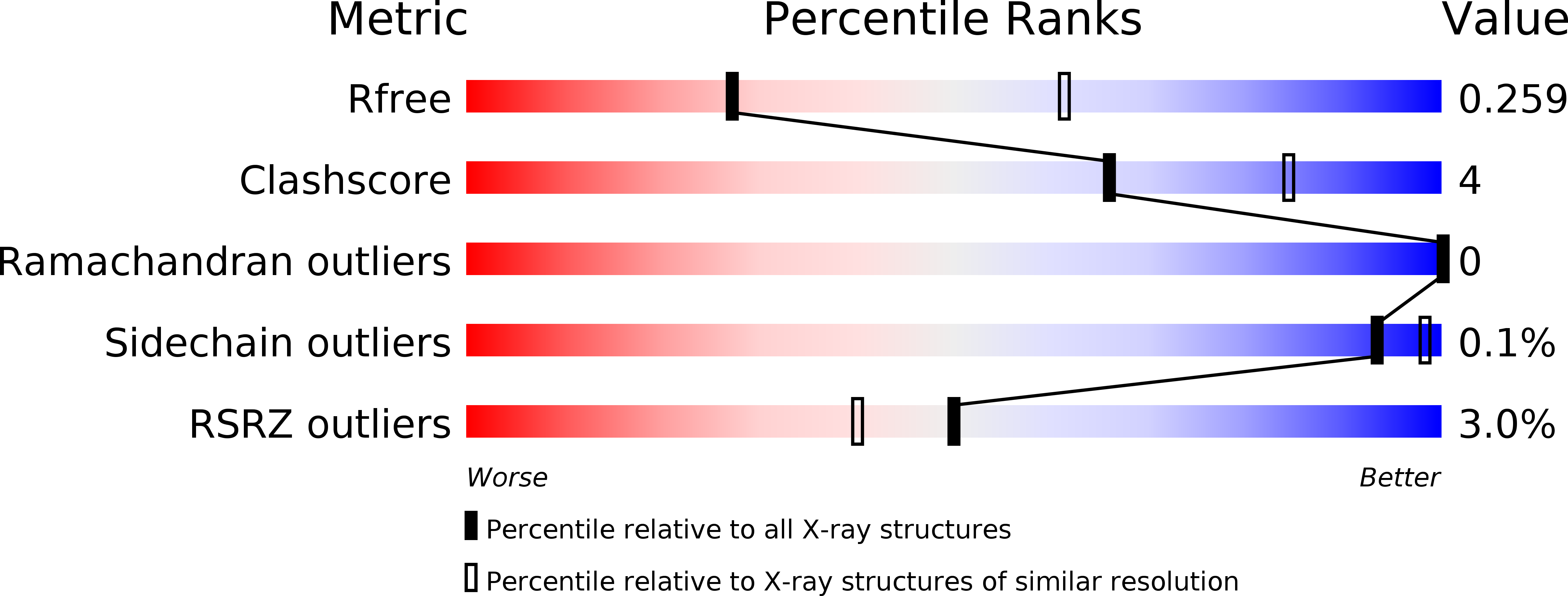
Position 123 of halohydrin dehalogenase HheG plays an important role in stability, activity, and enantioselectivity.
Solarczek, J., Klunemann, T., Brandt, F., Schrepfer, P., Wolter, M., Jacob, C.R., Blankenfeldt, W., Schallmey, A.(2019) Sci Rep 9: 5106-5106
- PubMed: 30911023
- DOI: https://doi.org/10.1038/s41598-019-41498-2
- Primary Citation of Related Structures:
6I9U, 6I9V, 6I9W - PubMed Abstract:
HheG from Ilumatobacter coccineus is a halohydrin dehalogenase with synthetically useful activity in the ring opening of cyclic epoxides with various small anionic nucleophiles. This enzyme provides access to chiral β-substituted alcohols that serve as building blocks in the pharmaceutical industry. Wild-type HheG suffers from low thermostability, which poses a significant drawback for potential applications. In an attempt to thermostabilize HheG by protein engineering, several single mutants at position 123 were identified which displayed up to 14 °C increased apparent melting temperatures and up to three-fold higher activity. Aromatic amino acids at position 123 resulted even in a slightly higher enantioselectivity. Crystal structures of variants T123W and T123G revealed a flexible loop opposite to amino acid 123. In variant T123G, this loop adopted two different positions resulting in an open or partially closed active site. Classical molecular dynamics simulations confirmed a high mobility of this loop. Moreover, in variant T123G this loop adopted a position much closer to residue 123 resulting in denser packing and increased buried surface area. Our results indicate an important role for position 123 in HheG and give first structural and mechanistic insight into the thermostabilizing effect of mutations T123W and T123G.
Organizational Affiliation:
Institute for Biochemistry, Biotechnology and Bioinformatics, Technische Universität Braunschweig, Spielmannstr. 7, 38106, Braunschweig, Germany.