Control by Metals of Staphylopine Dehydrogenase Activity during Metallophore Biosynthesis.
Hajjar, C., Fanelli, R., Laffont, C., Brutesco, C., Cullia, G., Tribout, M., Nurizzo, D., Borezee-Durant, E., Voulhoux, R., Pignol, D., Lavergne, J., Cavelier, F., Arnoux, P.(2019) J Am Chem Soc 141: 5555-5562
- PubMed: 30901200
- DOI: https://doi.org/10.1021/jacs.9b01676
- Primary Citation of Related Structures:
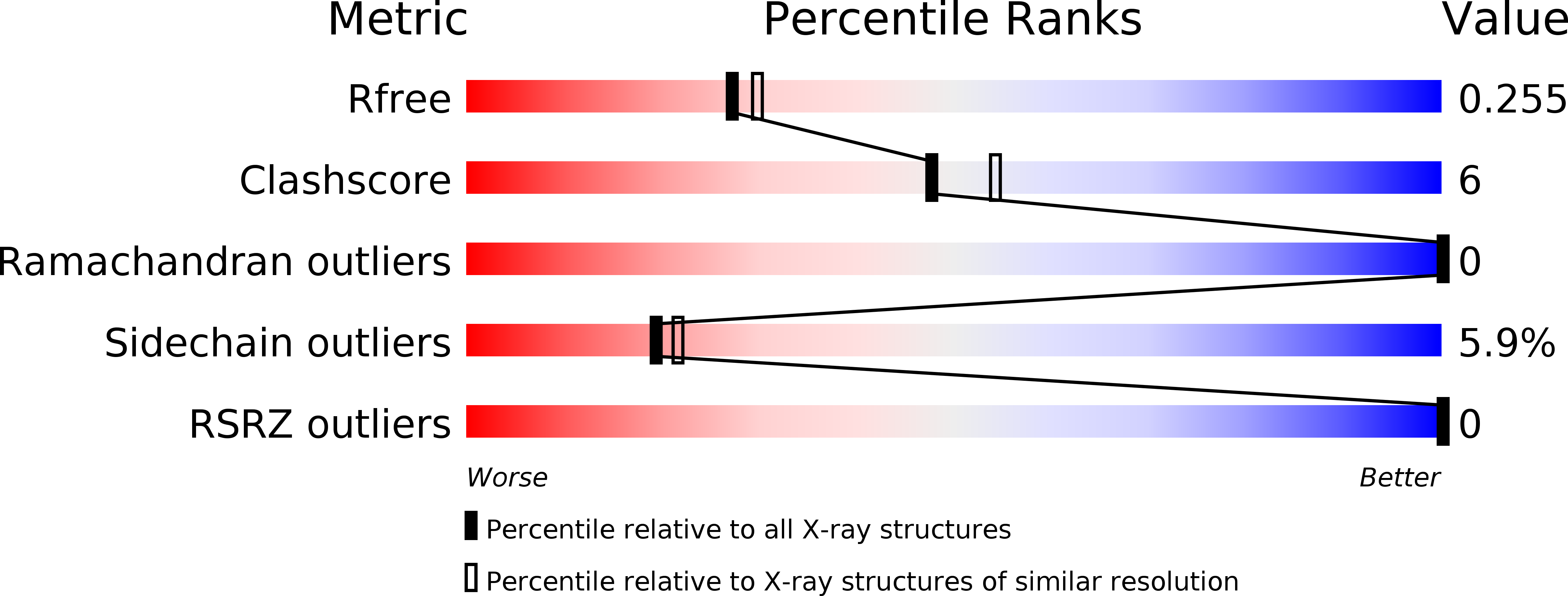
6GMZ, 6H31, 6H3D, 6H3F - PubMed Abstract:
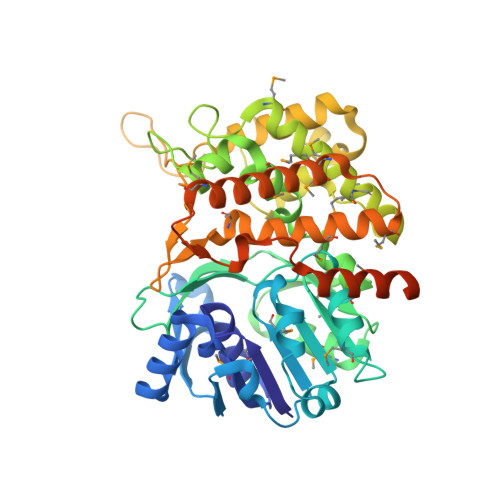
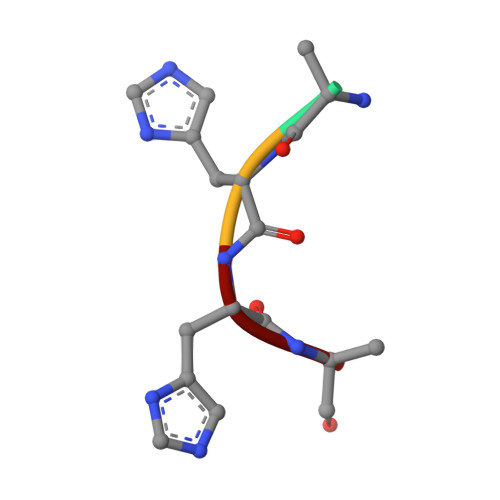
Enzymatic regulations are central processes for the adaptation to changing environments. In the particular case of metallophore-dependent metal uptake, there is a need to quickly adjust the production of these metallophores to the metal level outside the cell, to avoid metal shortage or overload, as well as waste of metallophores. In Staphylococcus aureus, CntM catalyzes the last biosynthetic step in the production of staphylopine, a broad-spectrum metallophore, through the reductive condensation of a pathway intermediate (xNA) with pyruvate. Here, we describe the chemical synthesis of this intermediate, which was instrumental in the structural and functional characterization of CntM and confirmed its opine synthase properties. The three-dimensional structure of CntM was obtained in an "open" form, in the apo state or as a complex with substrate or product. The xNA substrate appears mainly stabilized by its imidazole ring through a π-π interaction with the side chain of Tyr240. Intriguingly, we found that metals exerted various and sometime antagonistic effects on the reaction catalyzed by CntM: zinc and copper are moderate activators at low concentration and then total inhibitors at higher concentration, whereas manganese is only an activator and cobalt and nickel are only inhibitors. We propose a model in which the relative affinity of a metal toward xNA and an inhibitory binding site on the enzyme controls activation, inhibition, or both as a function of metal concentration. This metal-dependent regulation of a metallophore-producing enzyme might also take place in vivo, which could contribute to the adjustment of metallophore production to the internal metal level.
Organizational Affiliation:
Aix Marseille Université , CEA, CNRS, BIAM, F-13108 Saint Paul-Lez-Durance , France.