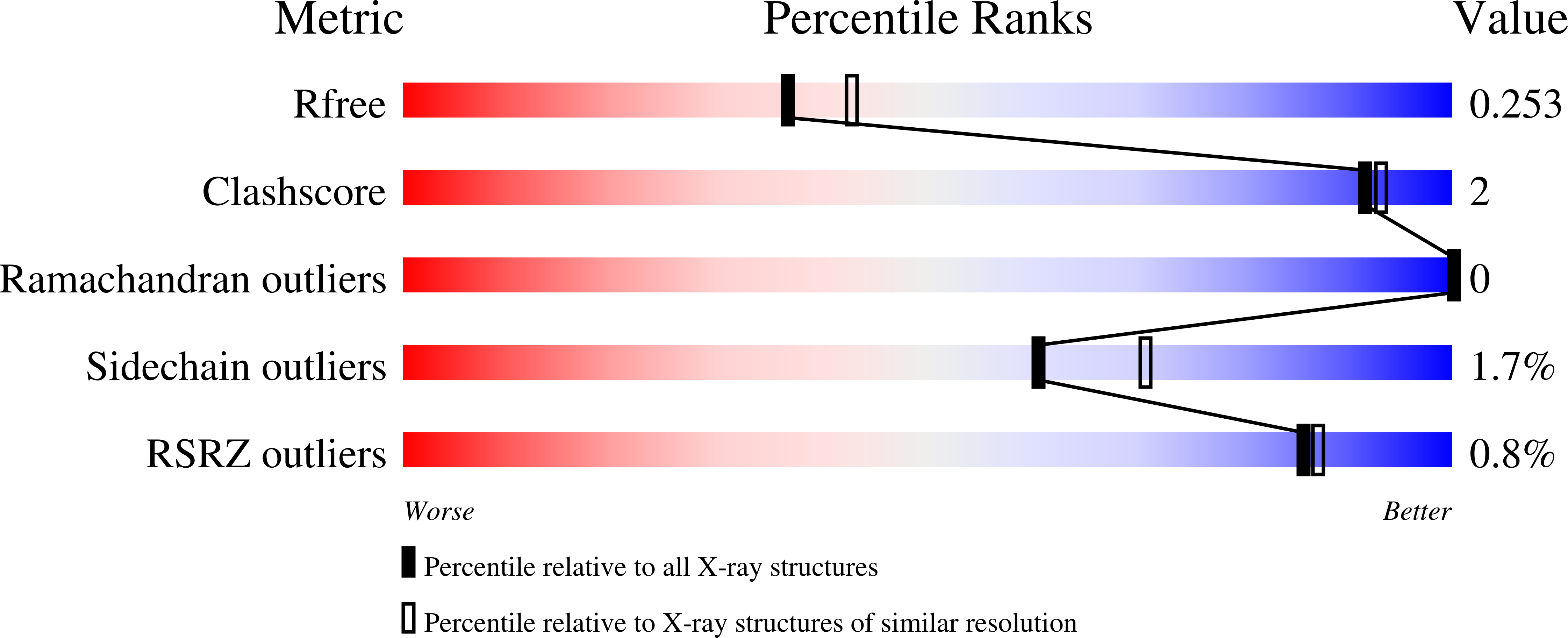
Specificity and mechanism of carbohydrate demethylation by cytochrome P450 monooxygenases.
Robb, C.S., Reisky, L., Bornscheuer, U.T., Hehemann, J.H.(2018) Biochem J 475: 3875-3886
- PubMed: 30404923
- DOI: https://doi.org/10.1042/BCJ20180762
- Primary Citation of Related Structures:
6G5O, 6G5Q - PubMed Abstract:
Degradation of carbohydrates by bacteria represents a key step in energy metabolism that can be inhibited by methylated sugars. Removal of methyl groups, which is critical for further processing, poses a biocatalytic challenge because enzymes need to overcome a high energy barrier. Our structural and computational analysis revealed how a member of the cytochrome P450 family evolved to oxidize a carbohydrate ligand. Using structural biology, we ascertained the molecular determinants of substrate specificity and revealed a highly specialized active site complementary to the substrate chemistry. Invariance of the residues involved in substrate recognition across the subfamily suggests that they are critical for enzyme function and when mutated, the enzyme lost substrate recognition. The structure of a carbohydrate-active P450 adds mechanistic insight into monooxygenase action on a methylated monosaccharide and reveals the broad conservation of the active site machinery across the subfamily.
Organizational Affiliation:
Max Planck Institute for Marine Microbiology, Celsiusstrasse 1, Bremen 28359, Germany.