Design of ultra-swollen lipidic mesophases for the crystallization of membrane proteins with large extracellular domains.
Zabara, A., Chong, J.T.Y., Martiel, I., Stark, L., Cromer, B.A., Speziale, C., Drummond, C.J., Mezzenga, R.(2018) Nat Commun 9: 544-544
- PubMed: 29416037
- DOI: https://doi.org/10.1038/s41467-018-02996-5
- Primary Citation of Related Structures:
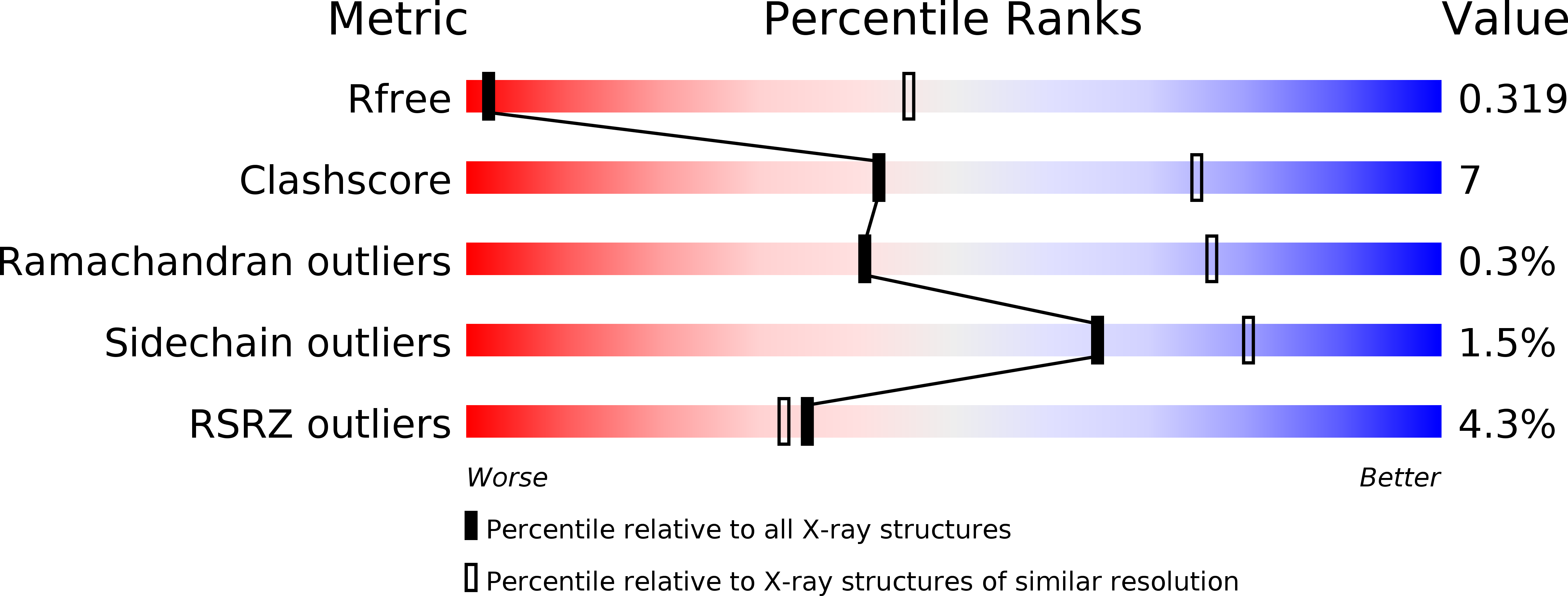
6F7A - PubMed Abstract:
In meso crystallization of membrane proteins from lipidic mesophases is central to protein structural biology but limited to membrane proteins with small extracellular domains (ECDs), comparable to the water channels (3-5 nm) of the mesophase. Here we present a strategy expanding the scope of in meso crystallization to membrane proteins with very large ECDs. We combine monoacylglycerols and phospholipids to design thermodynamically stable ultra-swollen bicontinuous cubic phases of double-gyroid (Ia3d), double-diamond (Pn3m), and double-primitive (Im3m) space groups, with water channels five times larger than traditional lipidic mesophases, and showing re-entrant behavior upon increasing hydration, of sequences Ia3d→Pn3m→Ia3d and Pn3m→Im3m→Pn3m, unknown in lipid self-assembly. We use these mesophases to crystallize membrane proteins with ECDs inaccessible to conventional in meso crystallization, demonstrating the methodology on the Gloeobacter ligand-gated ion channel (GLIC) protein, and show substantial modulation of packing, molecular contacts and activation state of the ensued proteins crystals, illuminating a general strategy in protein structural biology.
Organizational Affiliation:
Department of Health Sciences and Technology, ETH Zurich, Schmelzbergstrasse 9 LFO E23, 8092, Zürich, Switzerland.