Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide
Zhao, W.X.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Pectate lyase | 399 | Bacillus subtilis subsp. subtilis str. 168 | Mutation(s): 0 Gene Names: pel, BSU07560 EC: 4.2.2.2 | 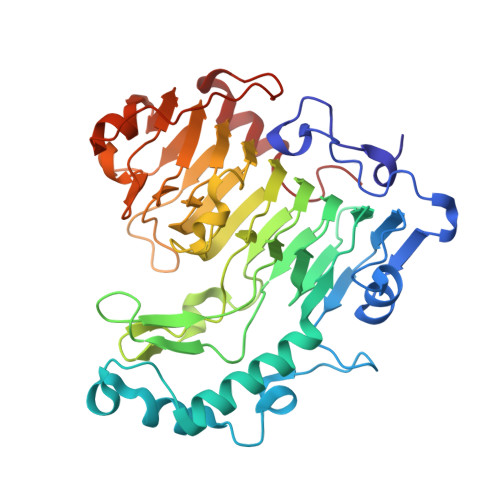 | |
UniProt | |||||
Find proteins for P39116 (Bacillus subtilis (strain 168)) Explore P39116 Go to UniProtKB: P39116 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P39116 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CA Query on CA | B [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| CL Query on CL | C [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 41.094 | α = 67.51 |
| b = 51.212 | β = 74.93 |
| c = 51.296 | γ = 78.75 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China | China | 31401638 |