Discovery of a Novel and Selective Indoleamine 2,3-Dioxygenase (IDO-1) Inhibitor 3-(5-Fluoro-1H-indol-3-yl)pyrrolidine-2,5-dione (EOS200271/PF-06840003) and Its Characterization as a Potential Clinical Candidate.
Crosignani, S., Bingham, P., Bottemanne, P., Cannelle, H., Cauwenberghs, S., Cordonnier, M., Dalvie, D., Deroose, F., Feng, J.L., Gomes, B., Greasley, S., Kaiser, S.E., Kraus, M., Negrerie, M., Maegley, K., Miller, N., Murray, B.W., Schneider, M., Soloweij, J., Stewart, A.E., Tumang, J., Torti, V.R., Van Den Eynde, B., Wythes, M.(2017) J Med Chem 60: 9617-9629
- PubMed: 29111717
- DOI: https://doi.org/10.1021/acs.jmedchem.7b00974
- Primary Citation of Related Structures:
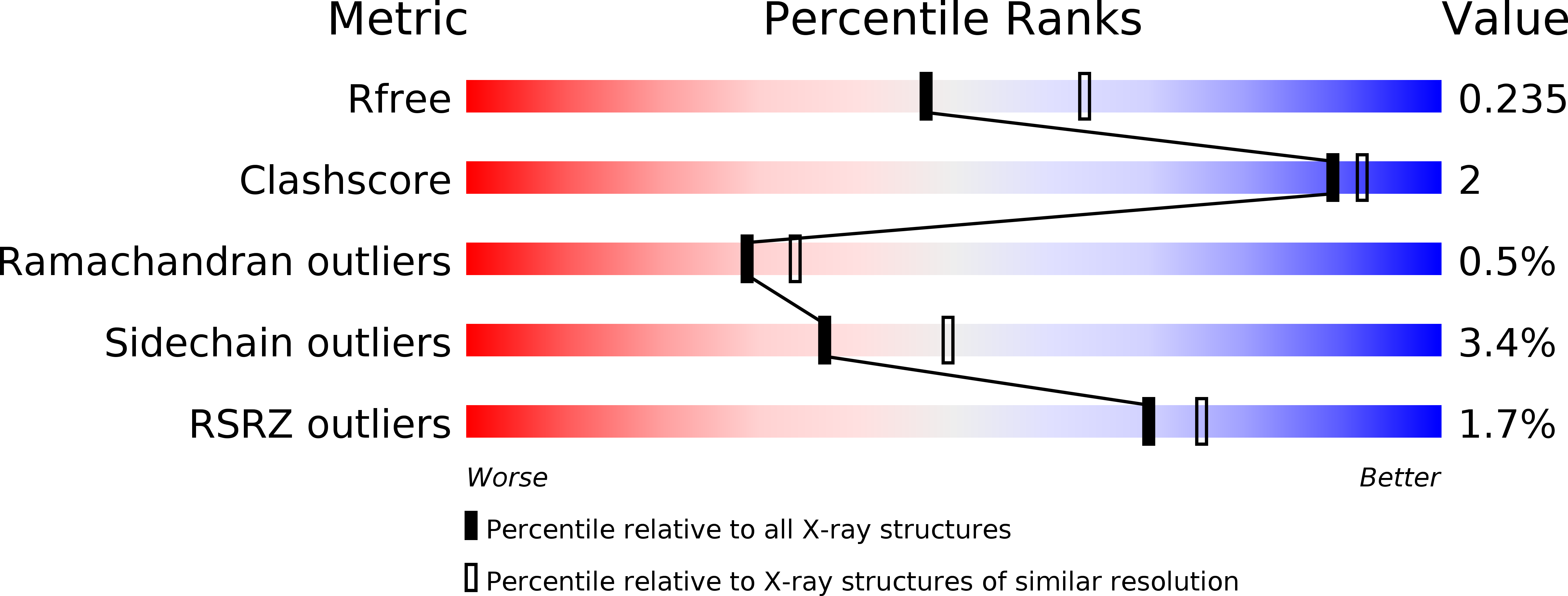
5WHR - PubMed Abstract:
Tumors use tryptophan-catabolizing enzymes such as indoleamine 2,3-dioxygenase (IDO-1) to induce an immunosuppressive environment. IDO-1 is induced in response to inflammatory stimuli and promotes immune tolerance through effector T-cell anergy and enhanced Treg function. As such, IDO-1 is a nexus for the induction of a key immunosuppressive mechanism and represents an important immunotherapeutic target in oncology. Starting from HTS hit 5, IDO-1 inhibitor 6 (EOS200271/PF-06840003) has been developed. The structure-activity relationship around 6 is described and rationalized using the X-ray crystal structure of 6 bound to human IDO-1, which shows that 6, differently from most of the IDO-1 inhibitors described so far, does not bind to the heme iron atom and has a novel binding mode. Clinical candidate 6 shows good potency in an IDO-1 human whole blood assay and also shows a very favorable ADME profile leading to favorable predicted human pharmacokinetic properties, including a predicted half-life of 16-19 h.
Organizational Affiliation:
iTeos Therapeutics , Rue des Frères Wright 29, 6041 Gosselies, Belgium.