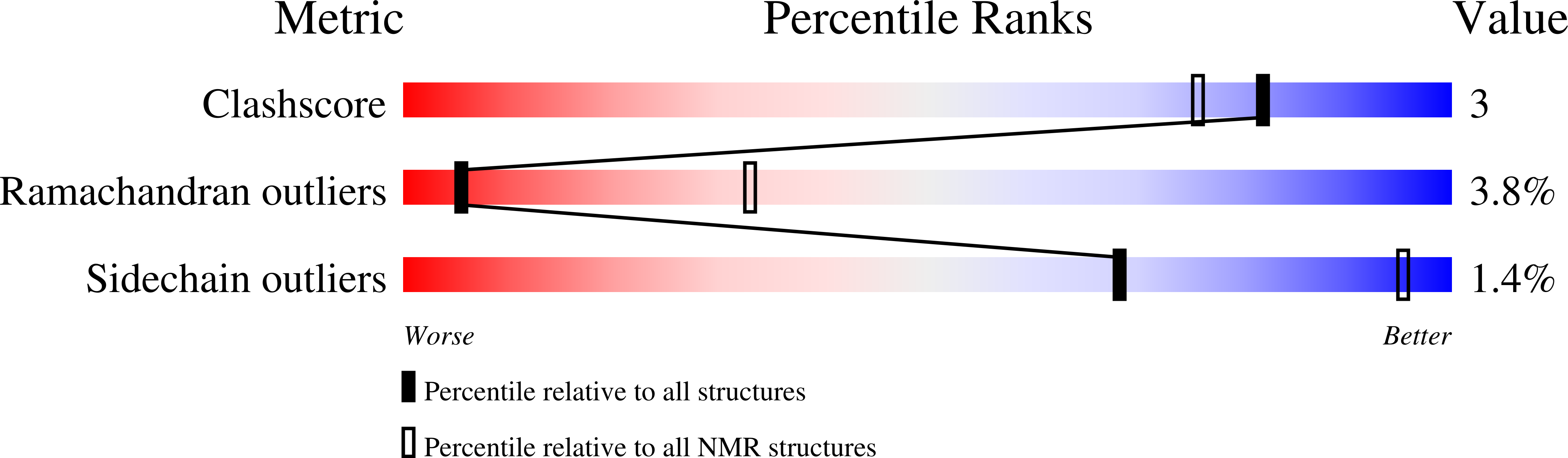Solution Structure and Expression Profile of an Insect Cytokine: Manduca sexta Stress Response Peptide-2.
Schrag, L.G., Cao, X., Herrera, A.I., Wang, Y., Jiang, H., Prakash, O.(2017) Protein Pept Lett 24: 3-11
- PubMed: 27903232
- DOI: https://doi.org/10.2174/0929866524666161121142840
- Primary Citation of Related Structures:
5W54 - PubMed Abstract:
Manduca sexta stress response peptide-2 (SRP2) is predicted to be a 25-residue peptide (FGVKDGKCPSGRVRRLGICVPDDDY), which may function as an insect cytokine to regulate immune responses. Produced as an inactive precursor, endogenous proSRP2 is probably converted to active SRP2 by limited proteolysis in response to invading pathogens, along with prophenoloxidase and pro-Spätzle activation. In addition to immunity, SRP2 may control head morphogenesis or other developmental processes in the lepidopteran insect. We have examined the profiles of SRP2 gene expression in terms of immune induction capacity, tissue specificity, and developmental changes. To gain insights into its functions, we chemically synthesized SRP2, injected the peptide solution into naïve larvae, and detected significant up-regulation of several antimicrobial peptide genes. We determined the 3D molecular structure in solution of SRP2 by two-dimensional 1H-1H NMR spectroscopy. SRP2 has an ordered structure, which is composed of two short β-strands at regions R12 - R15 and I18 - V20, one type-I' β-turn at region R15 - I18, and a half turn at region C8 - S10 in its welldefined core stabilized by a covalent disulfide bond between C8 and C19. The secondary and tertiary structures are further stabilized by hydrogen bonds. Possible relationships between the structure and function are also discussed.
Organizational Affiliation:
Department of Entomology and Plant Pathology, Oklahoma State University Stillwater, OK 74078, USA.