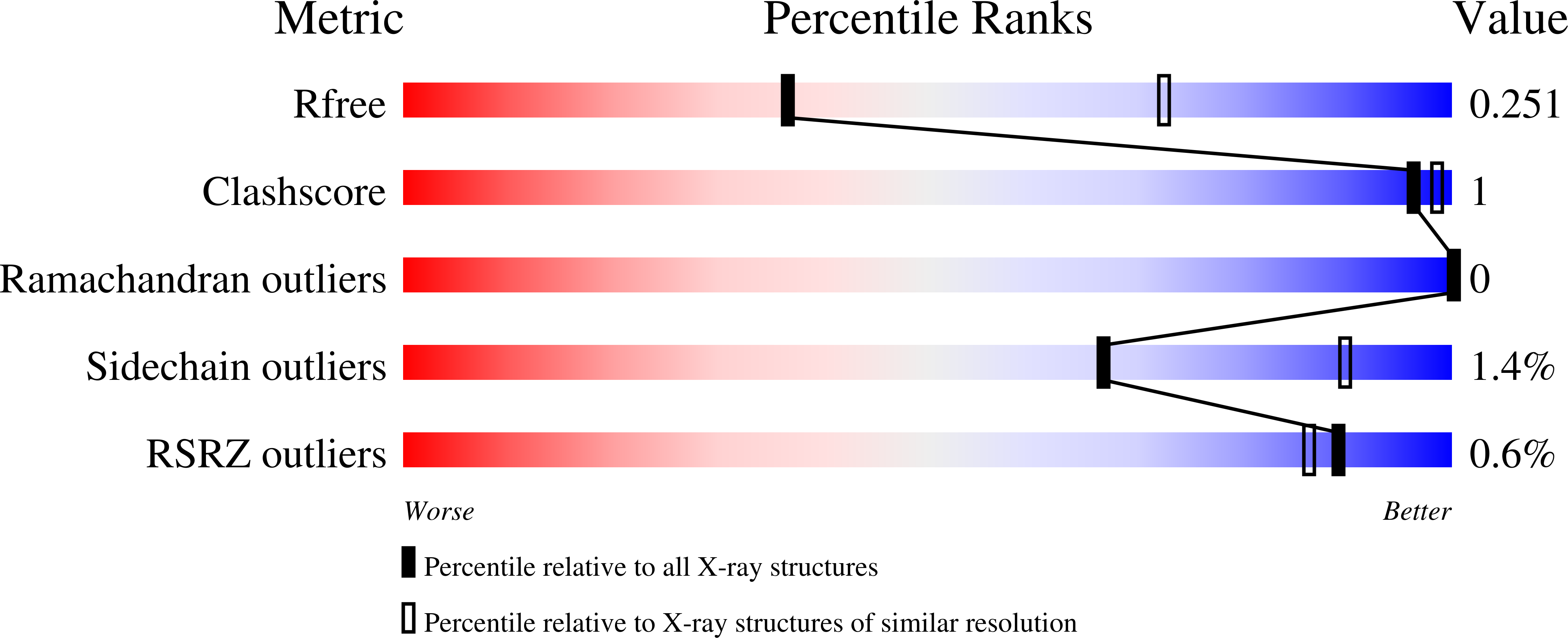
The C-terminal region of translesion synthesis DNA polymerase eta is partially unstructured and has high conformational flexibility.
Powers, K.T., Elcock, A.H., Washington, M.T.(2018) Nucleic Acids Res 46: 2107-2120
- PubMed: 29385534
- DOI: https://doi.org/10.1093/nar/gky031
- Primary Citation of Related Structures:
5VTP - PubMed Abstract:
Eukaryotic DNA polymerase η catalyzes translesion synthesis of thymine dimers and 8-oxoguanines. It is comprised of a polymerase domain and a C-terminal region, both of which are required for its biological function. The C-terminal region mediates interactions with proliferating cell nuclear antigen (PCNA) and other translesion synthesis proteins such as Rev1. This region contains a ubiquitin-binding/zinc-binding (UBZ) motif and a PCNA-interacting protein (PIP) motif. Currently little structural information is available for this region of polymerase η. Using a combination of approaches-including genetic complementation assays, X-ray crystallography, Langevin dynamics simulations, and small-angle X-ray scattering-we show that the C-terminal region is partially unstructured and has high conformational flexibility. This implies that the C-terminal region acts as a flexible tether linking the polymerase domain to PCNA thereby increasing its local concentration. Such tethering would facilitate the sampling of translesion synthesis polymerases to ensure that the most appropriate one is selected to bypass the lesion.
Organizational Affiliation:
Department of Biochemistry, University of Iowa College of Medicine, Iowa City, IA 52242-1109, USA.