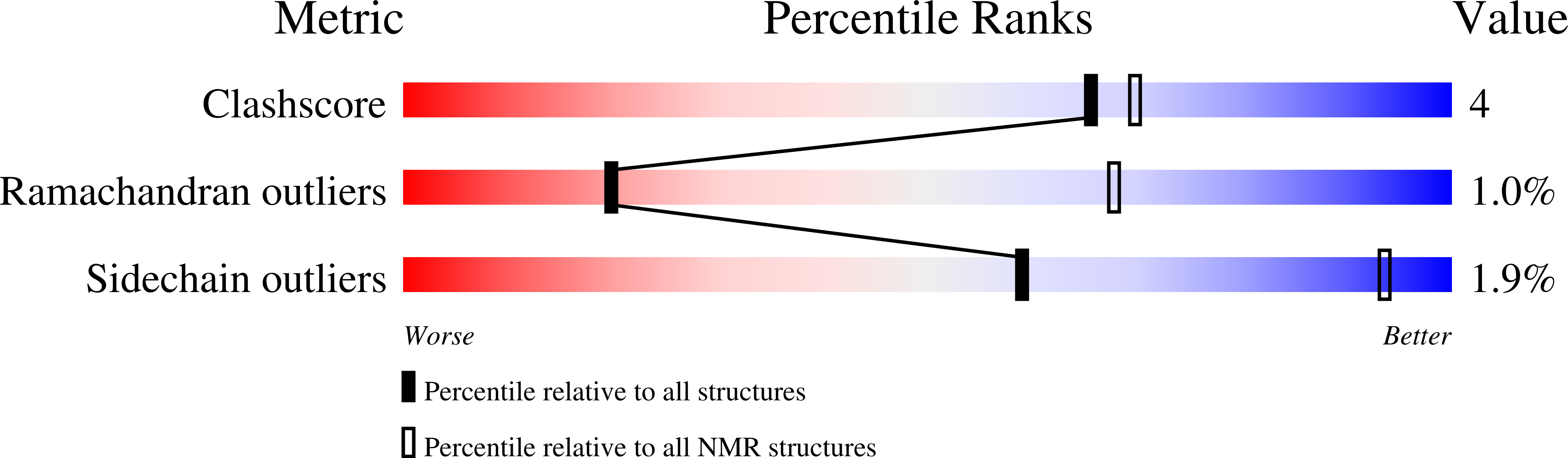Structure and interaction of Corynebacterium pseudotuberculosis cold shock protein A with Y-box single-stranded DNA fragment.
Caruso, I.P., Panwalkar, V., Coronado, M.A., Dingley, A.J., Cornelio, M.L., Willbold, D., Arni, R.K., Eberle, R.J.(2018) FEBS J 285: 372-390
- PubMed: 29197185
- DOI: https://doi.org/10.1111/febs.14350
- Primary Citation of Related Structures:
5O6F - PubMed Abstract:
Cold shock proteins (Csps) function to preserve cell viability at low temperatures by binding to nucleic acids and consequently control gene expression. The mesophilic bacterium Corynebacterium pseudotuberculosis is the causative agent of caseous lymphadenitis in animals, and infection in livestock is a considerable economic burden worldwide. In this report, the structure of cold shock protein A from Cp (Cp-CspA) and biochemical analysis of its temperature-dependent interaction with a Y-box ssDNA motif is presented. The Cp-CspA structure contains five β-strands making up a β-barrel fold with 11 hydrophobic core residues and two salt bridges that confers it with a melting temperature of ~ 54 °C that is similar to mesophilic Bs-CspB. Chemical shift perturbations analysis revealed that residues in the nucleic acid-binding motifs (RNP 1 and 2) and loop 3 are involved in binding to the Y-box fragment either by direct interaction or by conformational rearrangements remote from the binding region. Fluorescence quenching experiments of Cp-CspA showed that the dissociation constants for Y-box ssDNA binding is nanomolar and the binding affinity decreased as the temperature increased, indicating that the interaction is enthalpically driven and the hydrogen bonds and van der Waals forces are important contributions for complex stabilization. The Y31 of Cp-CspA is a particular occurrence among Csps from mesophilic bacteria that provide a possible explanation for the higher binding affinity to ssDNA than that observed for Bs-CspB. Anisotropy measurements indicated that the reduction in molecular mobility of Cp-CspA upon Y-box binding is characterized by a cooperative process.
Organizational Affiliation:
Department of Physics, Multiuser Center for Biomolecular Innovation (CMIB), IBILCE/UNESP, São José do Rio Preto, São Paulo, Brazil.