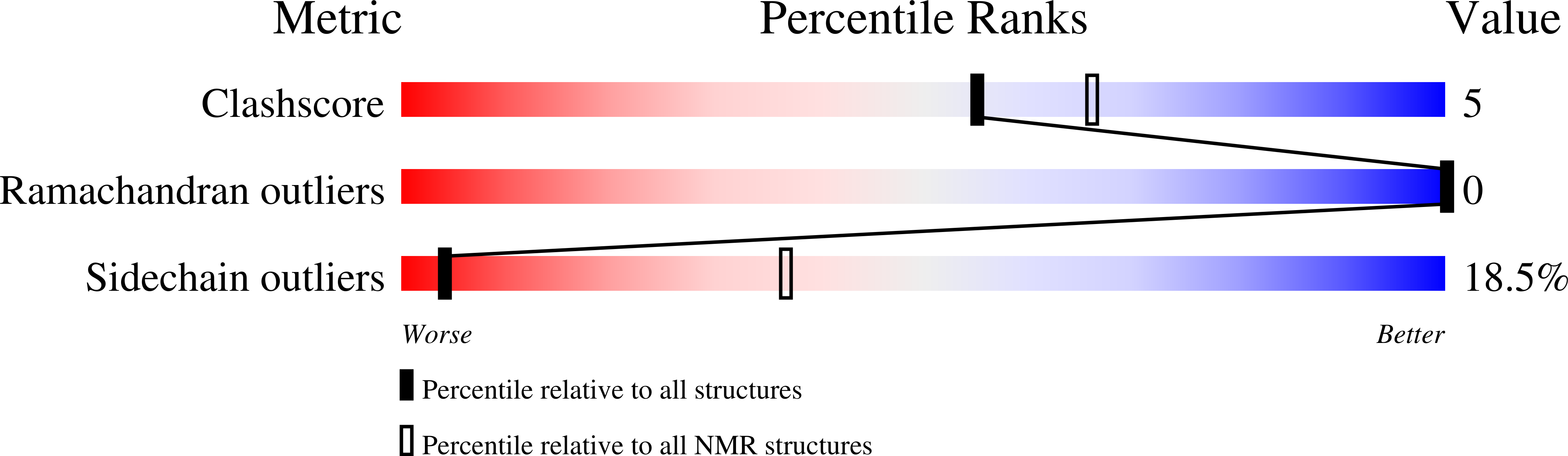Dimerization of the antimicrobial peptide arenicin plays a key role in the cytotoxicity but not in the antibacterial activity.
Panteleev, P.V., Myshkin, M.Y., Shenkarev, Z.O., Ovchinnikova, T.V.(2017) Biochem Biophys Res Commun 482: 1320-1326
- PubMed: 27940358
- DOI: https://doi.org/10.1016/j.bbrc.2016.12.035
- Primary Citation of Related Structures:
5M9U - PubMed Abstract:
The β-hairpin antimicrobial peptides arenicins from marine polychaeta Arenicola marina exhibit a broad spectrum of antimicrobial activity and high cytotoxicity. In this study the biological activities of arenicin-1 and its therapeutically valuable analog Ar-1[V8R] were investigated. The peptide Ar-1[V8R] displays significantly reduced cytotoxicity against mammalian cells relative to the wild-type arenicin-1. At the same time, both peptides exhibit similar antibacterial activities and kinetics of bacterial membrane permeabilization. Comparative NMR analysis of the peptides spatial structures in water and membrane-mimicking environment showed that Ar-1[V8R] in contrast to arenicin has significantly lower dimerization propensity. Thus, dimerization of the antimicrobial peptide arenicin plays a key role in the cytotoxicity but not in the antibacterial activity.
Organizational Affiliation:
M.M.Shemyakin and Yu.A. Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Miklukho-Maklaya str., 16/10, 117997 Moscow, Russia.