The Tp0684 (MglB-2) Lipoprotein of Treponema pallidum: A Glucose-Binding Protein with Divergent Topology.
Brautigam, C.A., Deka, R.K., Liu, W.Z., Norgard, M.V.(2016) PLoS One 11: e0161022-e0161022
- PubMed: 27536942
- DOI: https://doi.org/10.1371/journal.pone.0161022
- Primary Citation of Related Structures:
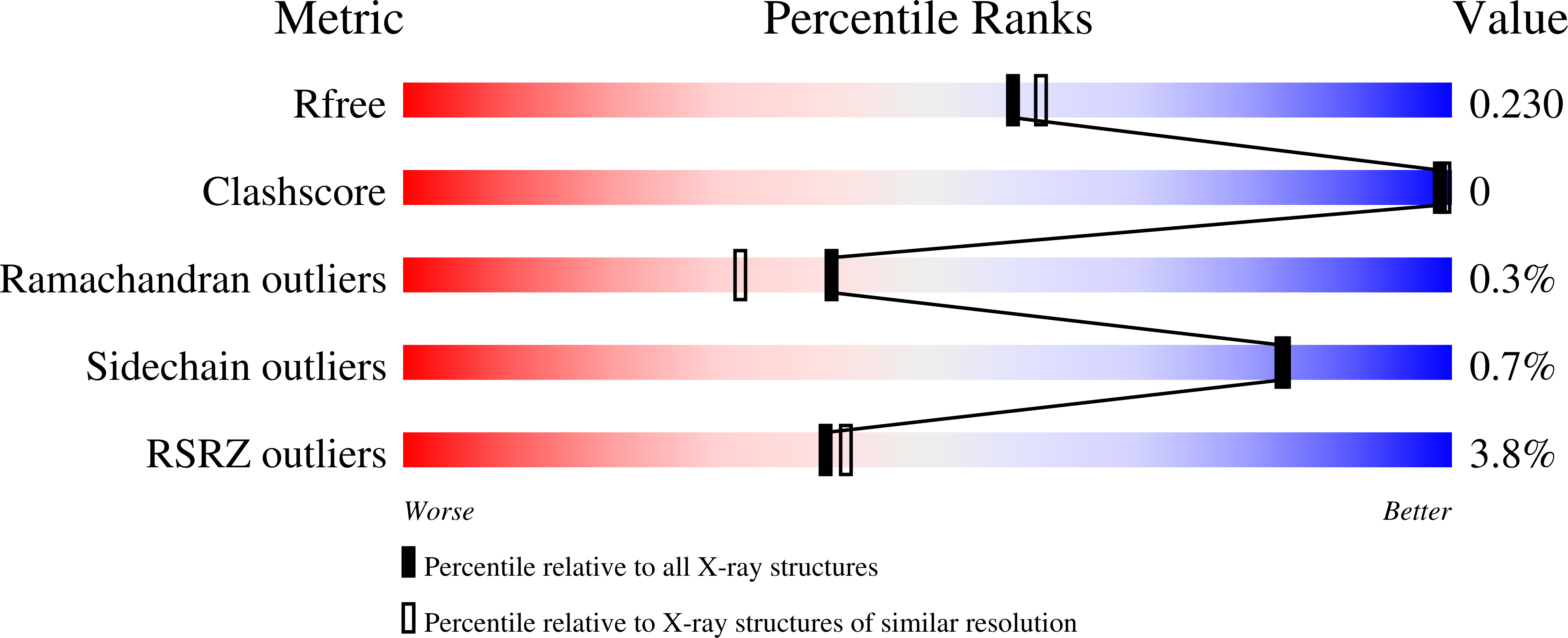
5JX2 - PubMed Abstract:
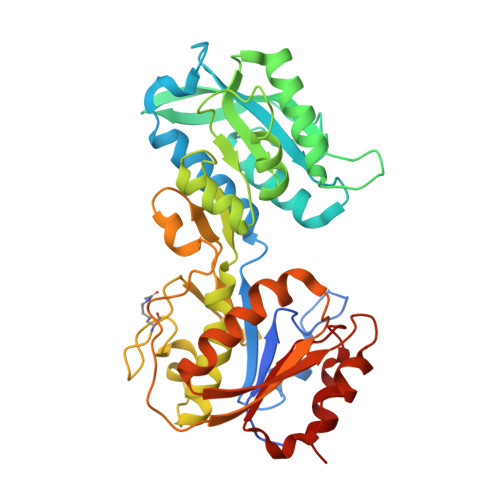
Treponema pallidum, the bacterium that causes syphilis, is an obligate human parasite. As such, it must acquire energy, in the form of carbon sources, from the host. There is ample evidence that the principal source of energy for this spirochete is D-glucose acquired from its environment, likely via an ABC transporter. Further, there is genetic evidence of a D-glucose chemotaxis system in T. pallidum. Both of these processes may be dependent on a single lipidated chemoreceptor: Tp0684, also called TpMglB-2 for its sequence homology to MglB of Escherichia coli. To broaden our understanding of this potentially vital protein, we determined a 2.05-Å X-ray crystal structure of a soluble form of the recombinant protein. Like its namesake, TpMglB-2 adopts a bilobed fold that is similar to that of the ligand-binding proteins (LBPs) of other ABC transporters. However, the protein has an unusual, circularly permuted topology. This feature prompted a series of biophysical studies that examined whether the protein's topological distinctiveness affected its putative chemoreceptor functions. Differential scanning fluorimetry and isothermal titration calorimetry were used to confirm that the protein bound D-glucose in a cleft between its two lobes. Additionally, analytical ultracentrifugation was employed to reveal that D-glucose binding is accompanied by a significant conformational change. TpMglB-2 thus appears to be fully functional in vitro, and given the probable central importance of the protein to T. pallidum's physiology, our results have implications for the viability and pathogenicity of this obligate human pathogen.
Organizational Affiliation:
Department of Biophysics, The University of Texas Southwestern Medical Center, Dallas, TX, 75390, United States of America.