Structural basis for substrate recognition and catalysis of a novel esterase E22 with a homoserine transacetylase-like fold
Zhang, Y., Yao, Q., Wang, P.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Esterase E22 | 370 | uncultured bacterium | Mutation(s): 0 | 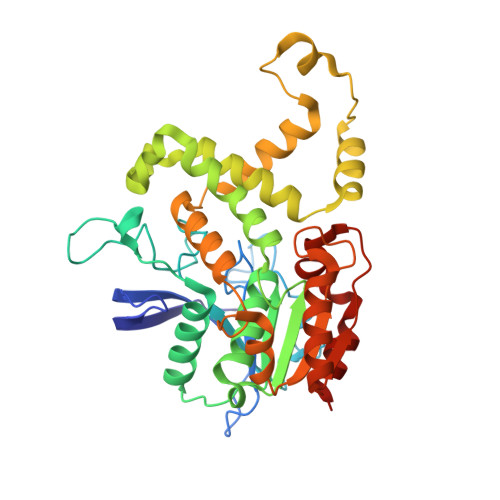 | |
UniProt | |||||
Find proteins for A0A1B1H1K0 (uncultured bacterium) Explore A0A1B1H1K0 Go to UniProtKB: A0A1B1H1K0 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A0A1B1H1K0 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.295 | α = 90 |
| b = 67.682 | β = 90 |
| c = 165.417 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| China | -- |