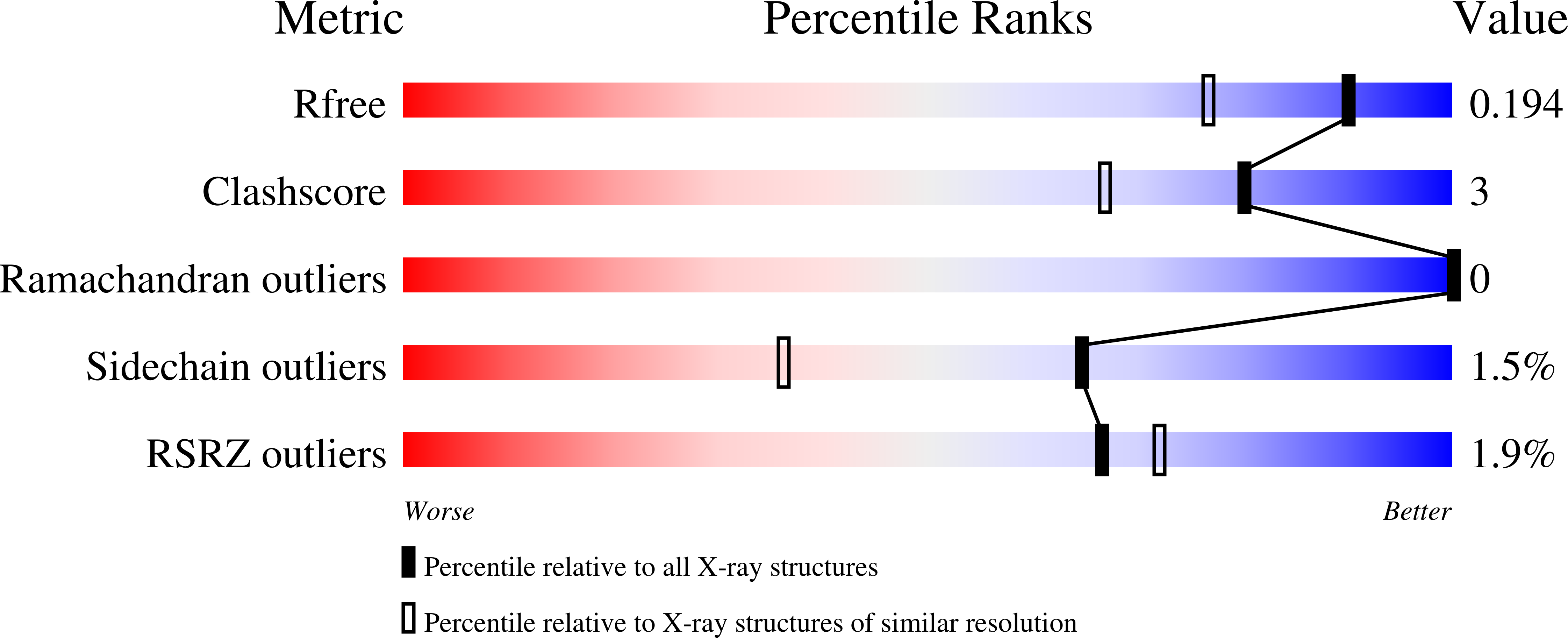
structure of dihydrofolate reductase from Yersinia pestis complex with
Chang, C., Maltseva, N., Kim, Y., Makowska-Grzyska, M., Mulligan, R., Papazisi, L., Anderson, W.F., Joachimiak, A., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.