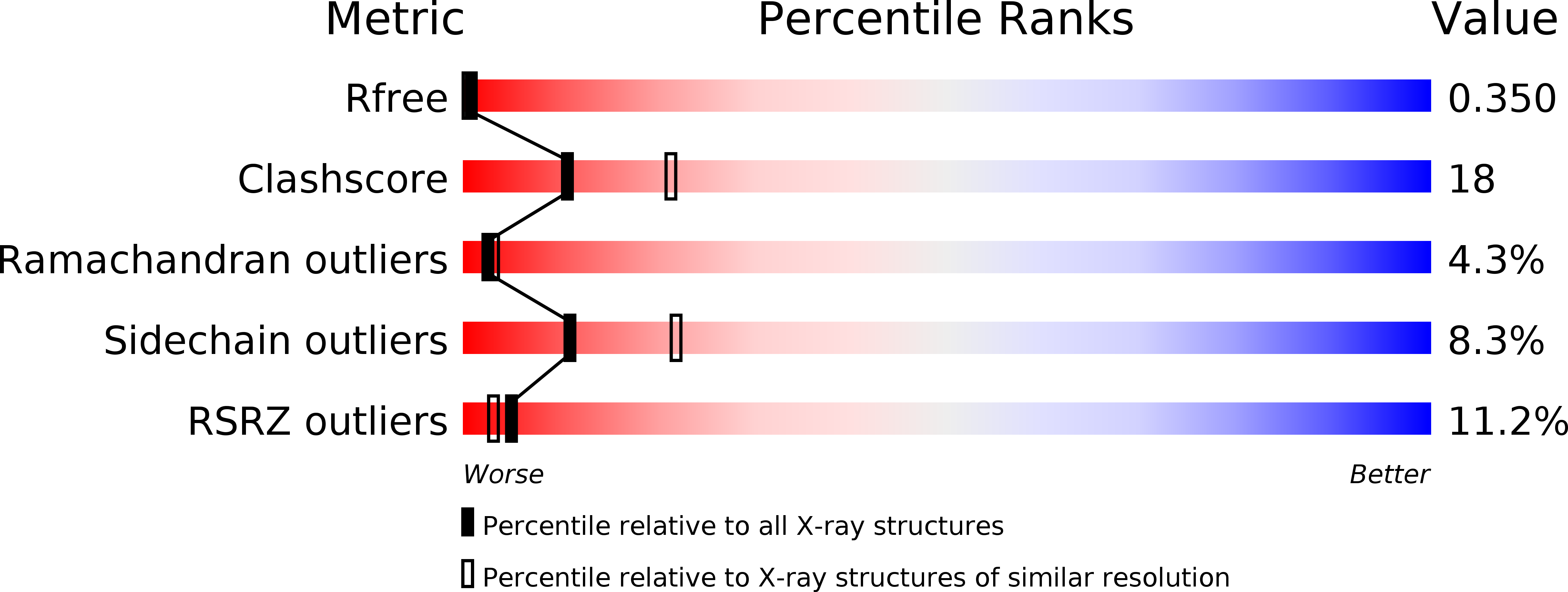
Suggested alternative starch utilization system from the human gut bacterium Bacteroides thetaiotaomicron.
Chaudet, M.M., Rose, D.R.(2016) Biochem Cell Biol 94: 241-246
- PubMed: 27093479
- DOI: https://doi.org/10.1139/bcb-2016-0002
- Primary Citation of Related Structures:
5DJW, 5F7C - PubMed Abstract:
The human digestive system is host to a highly populated ecosystem of bacterial species that significantly contributes to our assimilation of dietary carbohydrates. Bacteroides thetaiotaomicron is a member of this ecosystem, and participates largely in the role of the gut microbiome by breaking down dietary complex carbohydrates. This process of acquiring glycans from the colon lumen is predicted to rely on the mechanisms of proteins that are part of a classified system known as polysaccharide utilization loci (PUL). These loci are responsible for binding substrates at the cell outer membrane, internalizing them, and then hydrolyzing them within the periplasm into simple sugars. Here we report our investigation into specific components of a PUL, and suggest an alternative starch utilization system in B. thetaiotaomicron. Our analysis of an outer membrane binding protein, a SusD homolog, highlights its contribution to this PUL by acquiring starch-based sugars from the colon lumen. Through our structural characterization of two Family GH31 α-glucosidases, we reveal the flexibility of this bacterium with respect to utilizing a range of starch-derived glycans with an emphasis on branched substrates. With these results we demonstrate the predicted function of a gene locus that is capable of contributing to starch hydrolysis in the human colon.
Organizational Affiliation:
University of Waterloo, 200 University Avenue West, Waterloo, ON N2L 3G1, Canada.