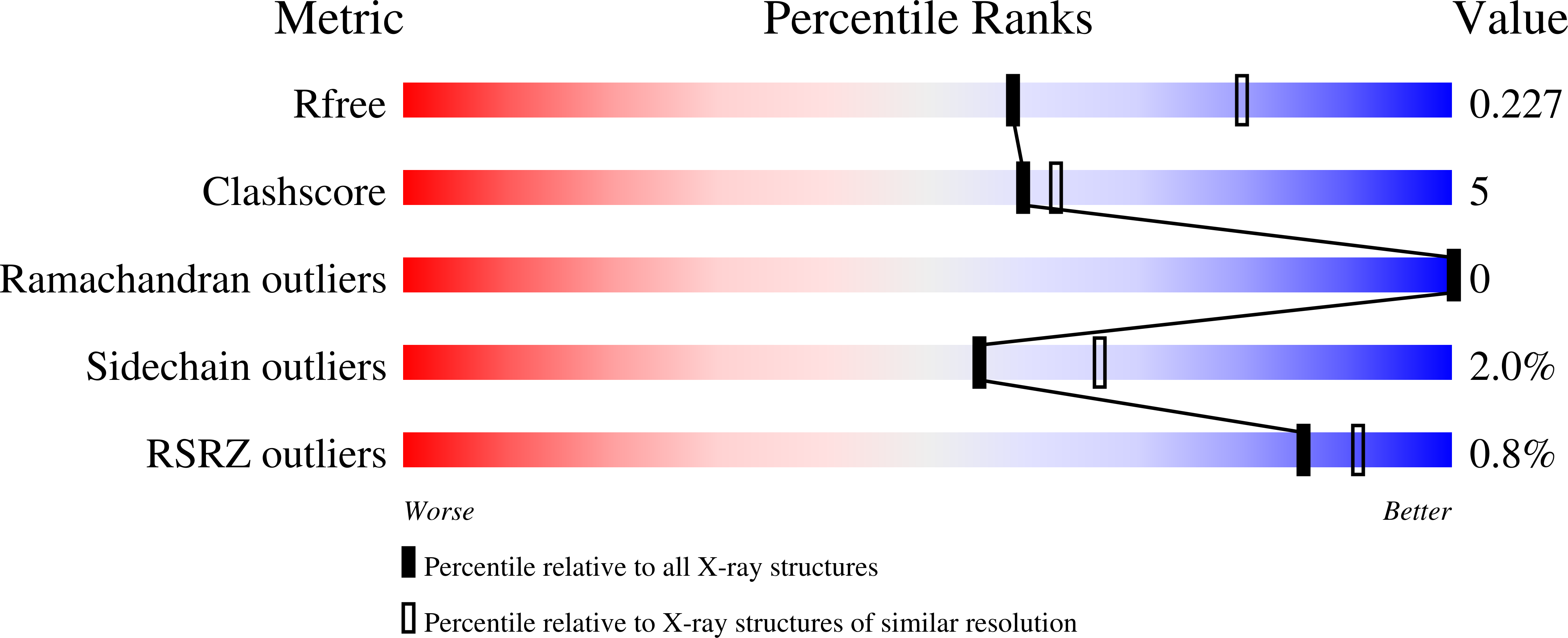
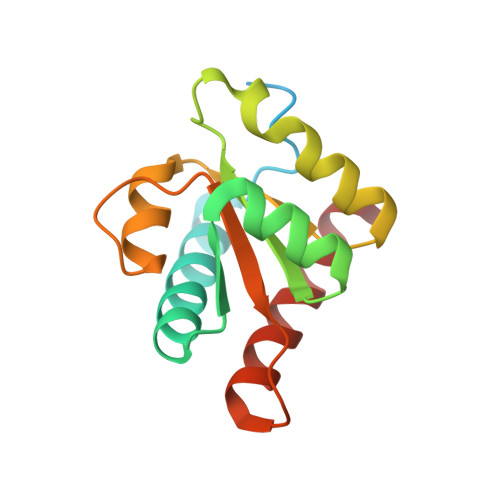
Conserved structure of Snu13 from the highly reduced spliceosome of Cyanidioschyzon merolae.
Black, C.S., Garside, E.L., MacMillan, A.M., Rader, S.D.(2016) Protein Sci 25: 911-916
- PubMed: 26833716
- DOI: https://doi.org/10.1002/pro.2894
- Primary Citation of Related Structures:
5EWR - PubMed Abstract:
Structural and functional analysis of proteins involved in pre-mRNA splicing is challenging because of the complexity of the splicing machinery, known as the spliceosome. Bioinformatic, proteomic, and biochemical analyses have identified a minimal spliceosome in the red alga Cyanidioschyzon merolae. This spliceosome consists of only 40 core proteins, compared to ∼ 70 in S. cerevisiae (yeast) and ∼ 150 in humans. We report the X-ray crystallographic analysis of C. merolae Snu13 (CmSnu13), a key component of the assembling spliceosome, and present evidence for conservation of Snu13 function in this algal splicing pathway. The near identity of CmSnu13's three-dimensional structure to yeast and human Snu13 suggests that C. merolae should be an excellent model system for investigating the structure and function of the conserved core of the spliceosome.
Organizational Affiliation:
Department of Chemistry, University of Northern British Columbia, Prince George, British Columbia, V2N 4Z9, Canada.