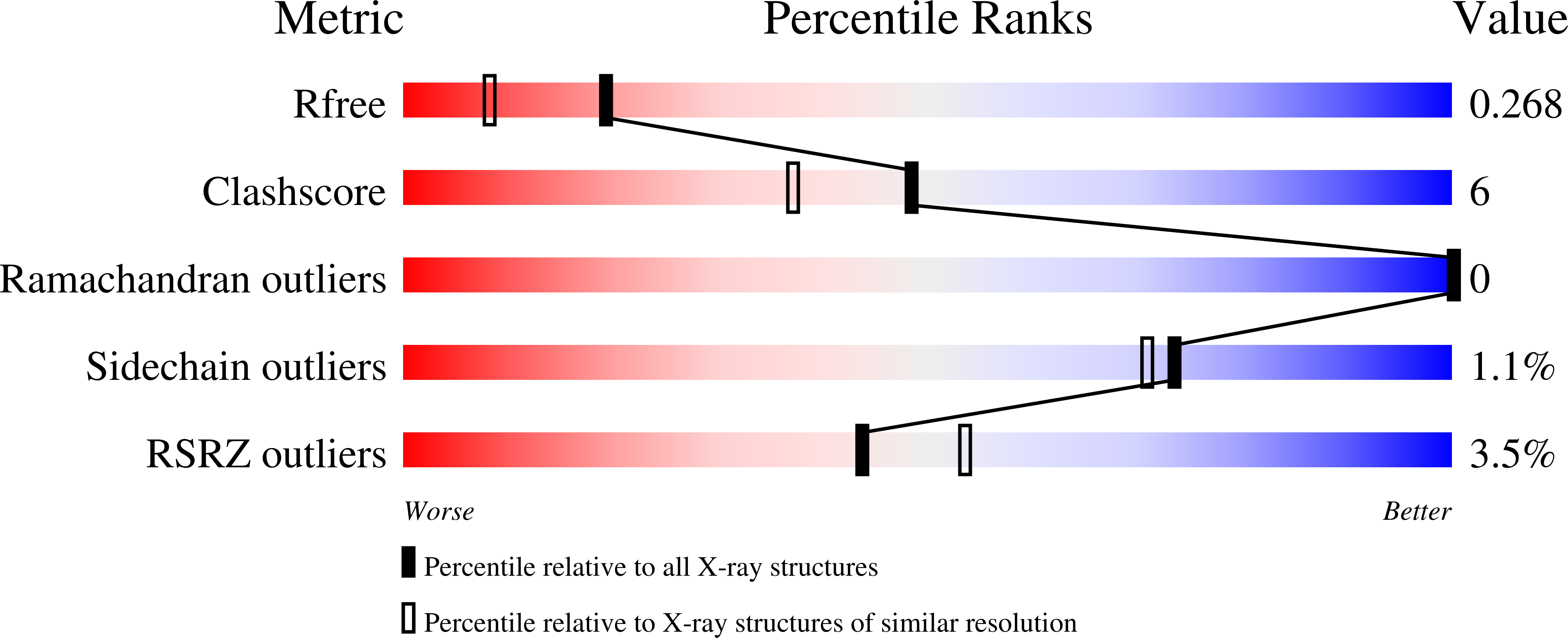
Crystal Structures of Trimethoprim-Resistant DfrA1 Rationalize Potent Inhibition by Propargyl-Linked Antifolates.
Lombardo, M.N., G-Dayanandan, N., Wright, D.L., Anderson, A.C.(2016) ACS Infect Dis 2: 149-156
- PubMed: 27624966
- DOI: https://doi.org/10.1021/acsinfecdis.5b00129
- Primary Citation of Related Structures:
5ECC, 5ECX - PubMed Abstract:
Multidrug-resistant Enterobacteriaceae, notably Escherichia coli and Klebsiella pneumoniae, have become major health concerns worldwide. Resistance to effective therapeutics is often carried by class I and II integrons that can confer insensitivity to carbapenems, extended spectrum β-lactamases, the antifolate trimethoprim, fluoroquinolones, and aminoglycosides. Specifically of interest to the study here, a prevalent gene (dfrA1) coding for an insensitive dihydrofolate reductase (DHFR) confers 190- or 1000-fold resistance to trimethoprim for K. pneumoniae and E. coli, respectively. Attaining inhibition of both the wild-type and resistant forms of the enzyme is critical for new antifolates. For several years, we have been developing the propargyl-linked antifolates (PLAs) as effective inhibitors against trimethoprim-resistant DHFR enzymes. Here, we show that the PLAs are active against both the wild-type and DfrA1 DHFR proteins. We report two high-resolution crystal structures of DfrA1 bound to potent PLAs. The structure-activity relationships and crystal structures will be critical in driving the design of broadly active inhibitors against wild-type and resistant DHFR.
Organizational Affiliation:
Department of Pharmaceutical Sciences, University of Connecticut , 69 North Eagleville Road, Storrs, Connecticut 06269, United States.