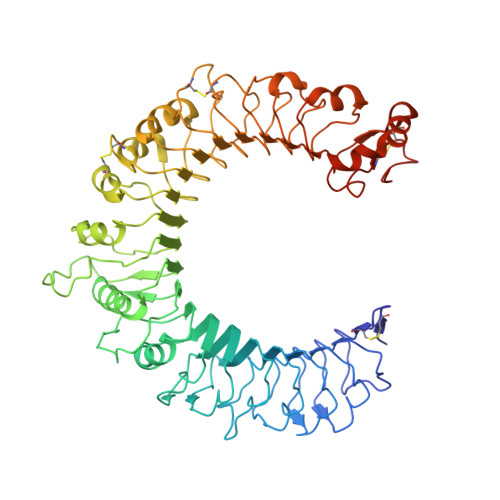
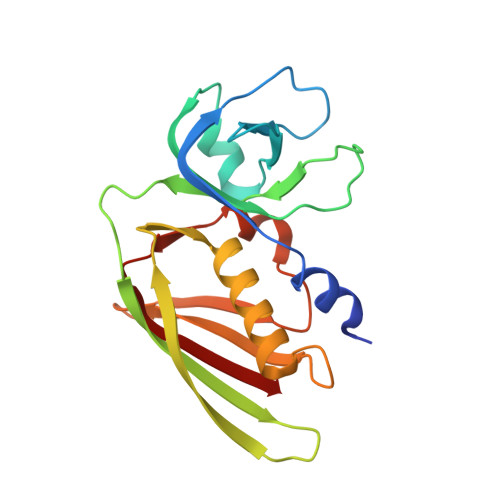
Structural basis for inhibition of TLR2 by staphylococcal superantigen-like protein 3 (SSL3).
Koymans, K.J., Feitsma, L.J., Brondijk, T.H., Aerts, P.C., Lukkien, E., Lossl, P., van Kessel, K.P., de Haas, C.J., van Strijp, J.A., Huizinga, E.G.(2015) Proc Natl Acad Sci U S A 112: 11018-11023
- PubMed: 26283364
- DOI: https://doi.org/10.1073/pnas.1502026112
- Primary Citation of Related Structures:
5D3D, 5D3I - PubMed Abstract:
Toll-like receptors (TLRs) are crucial in innate recognition of invading micro-organisms and their subsequent clearance. Bacteria are not passive bystanders and have evolved complex evasion mechanisms. Staphylococcus aureus secretes a potent TLR2 antagonist, staphylococcal superantigen-like protein 3 (SSL3), which prevents receptor stimulation by pathogen-associated lipopeptides. Here, we present crystal structures of SSL3 and its complex with TLR2. The structure reveals that formation of the specific inhibitory complex is predominantly mediated by hydrophobic contacts between SSL3 and TLR2 and does not involve interaction of TLR2-glycans with the conserved Lewis(X) binding site of SSL3. In the complex, SSL3 partially covers the entrance to the lipopeptide binding pocket in TLR2, reducing its size by ∼50%. We show that this is sufficient to inhibit binding of agonist Pam2CSK4 effectively, yet allows SSL3 to bind to an already formed TLR2-Pam2CSK4 complex. The binding site of SSL3 overlaps those of TLR2 dimerization partners TLR1 and TLR6 extensively. Combined, our data reveal a robust dual mechanism in which SSL3 interferes with TLR2 activation at two stages: by binding to TLR2, it blocks ligand binding and thus inhibits activation. Second, by interacting with an already formed TLR2-lipopeptide complex, it prevents TLR heterodimerization and downstream signaling.
Organizational Affiliation:
Department of Medical Microbiology, University Medical Center Utrecht, NL-3584 CX, Utrecht, The Netherlands;