Structural and Functional Insights Into Escherichia Coli Alpha2- Macroglobulin Endopeptidase Snap-Trap Inhibition.
Garcia-Ferrer, I., Arede, P., Gomez-Blanco, J., Luque, D., Duquerroy, S., Caston, J.R., Goulas, T., Gomis-Ruth, F.X.(2015) Proc Natl Acad Sci U S A 112: 8290
- PubMed: 26100869
- DOI: https://doi.org/10.1073/pnas.1506538112
- Primary Citation of Related Structures:
4ZIQ, 4ZIU, 4ZJG, 4ZJH, 5A42 - PubMed Abstract:
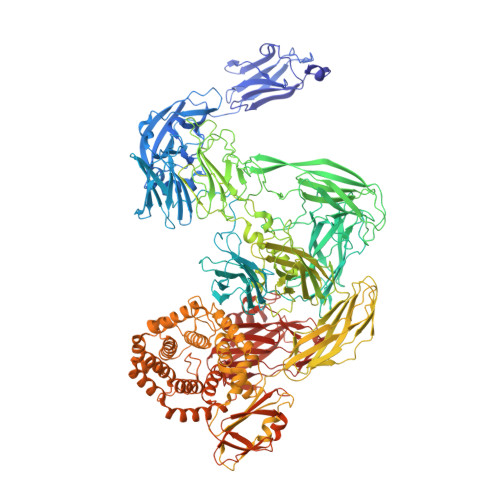
The survival of commensal bacteria requires them to evade host peptidases. Gram-negative bacteria from the human gut microbiome encode a relative of the human endopeptidase inhibitor, α2-macroglobulin (α2M). Escherichia coli α2M (ECAM) is a ∼ 180-kDa multidomain membrane-anchored pan-peptidase inhibitor, which is cleaved by host endopeptidases in an accessible bait region. Structural studies by electron microscopy and crystallography reveal that this cleavage causes major structural rearrangement of more than half the 13-domain structure from a native to a compact induced form. It also exposes a reactive thioester bond, which covalently traps the peptidase. Subsequently, peptidase-laden ECAM is shed from the membrane and may dimerize. Trapped peptidases are still active except against very large substrates, so inhibition potentially prevents damage of large cell envelope components, but not host digestion. Mechanistically, these results document a novel monomeric "snap trap."
Organizational Affiliation:
Proteolysis Lab, Department of Structural Biology ("María de Maeztu" Unit of Excellence), Molecular Biology Institute of Barcelona, Spanish Research Council (CSIC), 08028 Barcelona, Spain;