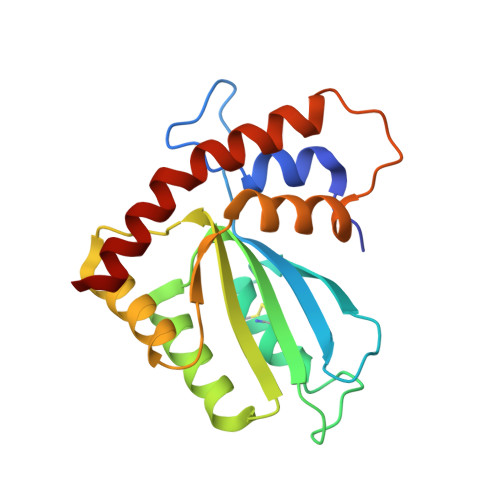
Structural basis of chimpanzee APOBEC3H dimerization stabilized by double-stranded RNA.
Matsuoka, T., Nagae, T., Ode, H., Awazu, H., Kurosawa, T., Hamano, A., Matsuoka, K., Hachiya, A., Imahashi, M., Yokomaku, Y., Watanabe, N., Iwatani, Y.(2018) Nucleic Acids Res 46: 10368-10379
- PubMed: 30060196
- DOI: https://doi.org/10.1093/nar/gky676
- Primary Citation of Related Structures:
5Z98 - PubMed Abstract:
APOBEC3H (A3H) is a mammal-specific cytidine deaminase that potently restricts the replication of retroviruses. Primate A3Hs are known to exert key selective pressures against the cross-species transmission of primate immunodeficiency viruses from chimpanzees to humans. Despite recent advances, the molecular structures underlying the functional mechanisms of primate A3Hs have not been fully understood. Here, we reveal the 2.20-Å crystal structure of the chimpanzee A3H (cpzA3H) dimer bound to a short double-stranded RNA (dsRNA), which appears to be similar to two recently reported structures of pig-tailed macaque A3H and human A3H. In the structure, the dsRNA-binding interface forms a specialized architecture with unique features. The analysis of the dsRNA nucleotides in the cpzA3H complex revealed the GC-rich palindrome-like sequence preference for dsRNA interaction, which is largely determined by arginine residues in loop 1. In cells, alterations of the cpzA3H residues critical for the dsRNA interaction severely reduce intracellular protein stability due to proteasomal degradation. This suggests that cpzA3H stability is regulated by the dsRNA-mediated dimerization as well as by unknown cellular machinery through proteasomal degradation in cells. Taken together, these findings highlight unique structural features of primate A3Hs that are important to further understand their cellular functions and regulation.
Organizational Affiliation:
Clinical Research Center, National Hospital Organization Nagoya Medical Center, Nagoya, Aichi 460-0001, Japan.