Catalytic mechanism of tyrosinase implied from the quinone formation on the Tyr98 residue of the caddie protein
Matoba, Y., Sugiyama, M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Tyrosinase | 281 | Streptomyces castaneoglobisporus | Mutation(s): 1 Gene Names: tyrC EC: 1.14.18.1 | 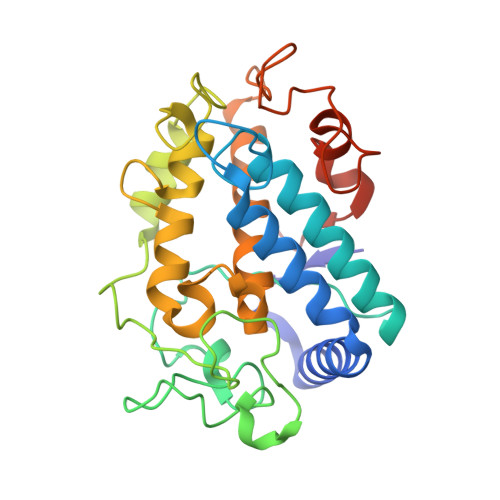 | |
UniProt | |||||
Find proteins for Q83WS2 (Streptomyces castaneoglobisporus) Explore Q83WS2 Go to UniProtKB: Q83WS2 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q83WS2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MelC | 134 | Streptomyces castaneoglobisporus | Mutation(s): 0 Gene Names: orf378 | 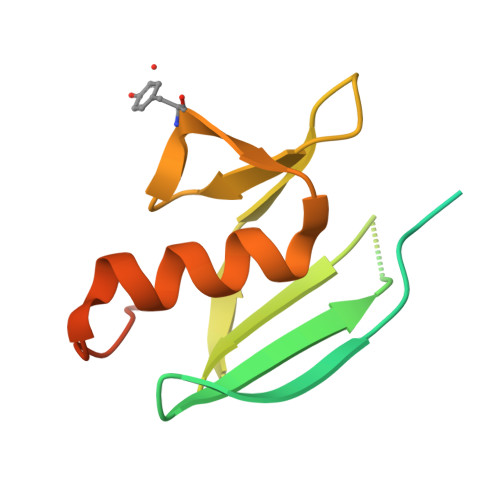 | |
UniProt | |||||
Find proteins for Q83WS1 (Streptomyces castaneoglobisporus) Explore Q83WS1 Go to UniProtKB: Q83WS1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q83WS1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CU Query on CU | C [auth A], D [auth A], I [auth B] | COPPER (II) ION Cu JPVYNHNXODAKFH-UHFFFAOYSA-N |  | ||
| NO3 Query on NO3 | E [auth A] F [auth A] G [auth A] H [auth A] J [auth B] | NITRATE ION N O3 NHNBFGGVMKEFGY-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| DAH Query on DAH | B | L-PEPTIDE LINKING | C9 H11 N O4 |  | PHE |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 64.91 | α = 90 |
| b = 97.35 | β = 90 |
| c = 54.92 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SHELXL-97 | refinement |
| CNS | phasing |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| Funding Organization | Location | Grant Number |
|---|---|---|
| KAKENHI | Japan | 25109530, 15H009470 |