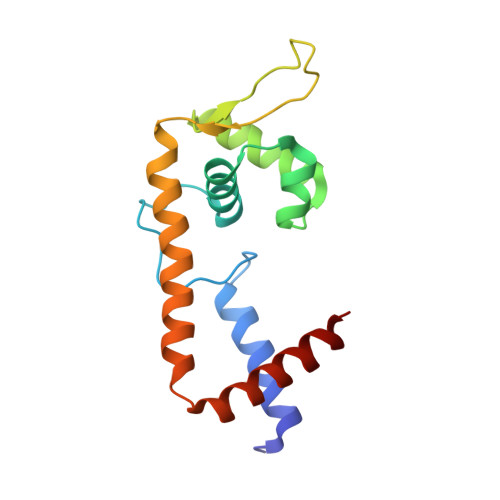
Allosteric histidine switch for regulation of intracellular zinc(II) fluctuation.
Zhu, R., Song, Y., Liu, H., Yang, Y., Wang, S., Yi, C., Chen, P.R.(2017) Proc Natl Acad Sci U S A 114: 13661-13666
- PubMed: 29229866
- DOI: https://doi.org/10.1073/pnas.1708563115
- Primary Citation of Related Structures:
5YHX, 5YHY, 5YHZ, 5YI0, 5YI1, 5YI2, 5YI3 - PubMed Abstract:
Metalloregulators allosterically control transcriptional activity through metal binding-induced reorganization of ligand residues and/or hydrogen bonding networks, while the coordination atoms on the same ligand residues remain seldom changed. Here we show that the MarR-type zinc transcriptional regulator ZitR switches one of its histidine nitrogen atoms for zinc coordination during the allosteric control of DNA binding. The Zn(II)-coordination nitrogen on histidine 42 within ZitR's high-affinity zinc site (site 1) switches from Nε2 to Nδ1 upon Zn(II) binding to its low-affinity zinc site (site 2), which facilitates ZitR's conversion from the nonoptimal to the optimal DNA-binding conformation. This histidine switch-mediated cooperation between site 1 and site 2 enables ZitR to adjust its DNA-binding affinity in response to a broad range of zinc fluctuation, which may allow the fine tuning of transcriptional regulation.
Organizational Affiliation:
Synthetic and Functional Biomolecules Center, Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China.