How electrostatic networks modulate specificity and stability of collagen.
Zheng, H., Lu, C., Lan, J., Fan, S., Nanda, V., Xu, F.(2018) Proc Natl Acad Sci U S A 115: 6207-6212
- PubMed: 29844169
- DOI: https://doi.org/10.1073/pnas.1802171115
- Primary Citation of Related Structures:
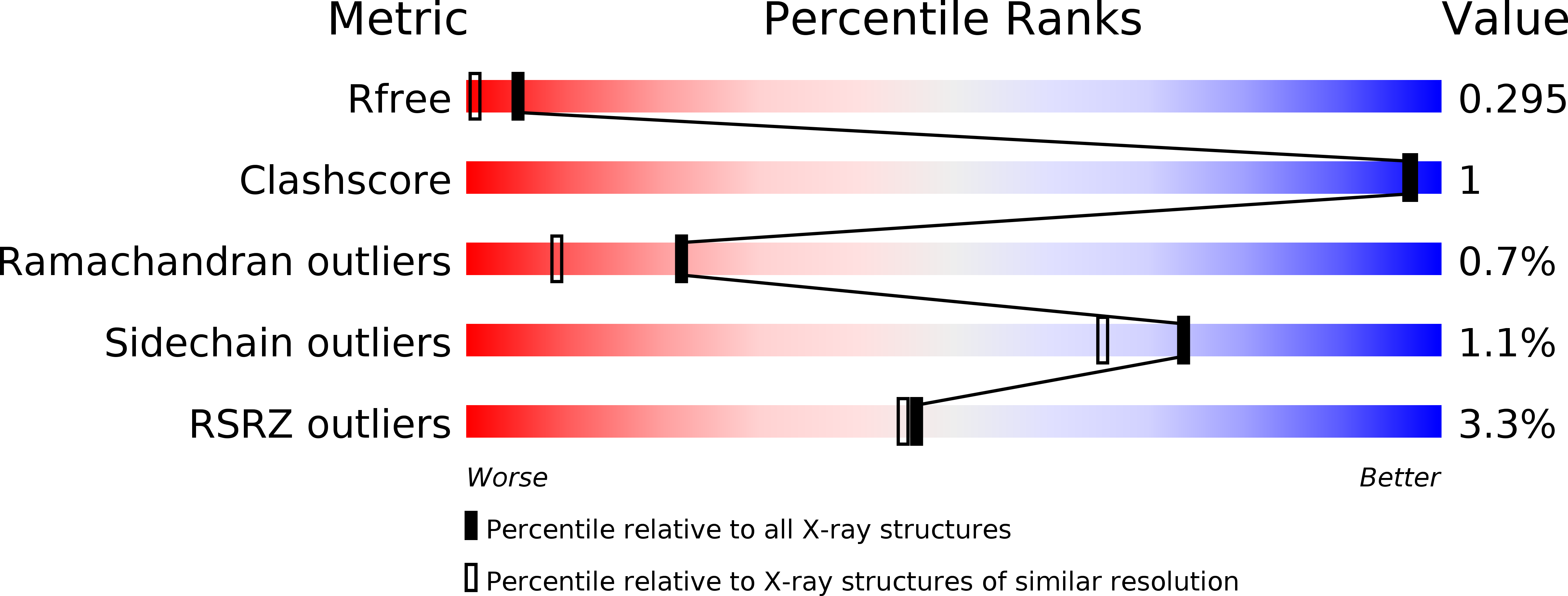
5YAN - PubMed Abstract:
One-quarter of the 28 types of natural collagen exist as heterotrimers. The oligomerization state of collagen affects the structure and mechanics of the extracellular matrix, providing essential cues to modulate biological and pathological processes. A lack of high-resolution structural information limits our mechanistic understanding of collagen heterospecific self-assembly. Here, the 1.77-Å resolution structure of a synthetic heterotrimer demonstrates the balance of intermolecular electrostatics and hydrogen bonding that affects collagen stability and heterospecificity of assembly. Atomistic simulations and mutagenesis based on the solved structure are used to explore the contributions of specific interactions to energetics. A predictive model of collagen stability and specificity is developed for engineering novel collagen structures.
Organizational Affiliation:
Ministry of Education Key Laboratory of Carbohydrate Chemistry and Biotechnology, School of Biotechnology, Jiangnan University, 214122 Wuxi, China.