Mechanistic and Structural Insights Into the Unique TetR-Dependent Regulation of a Drug Efflux Pump inMycobacterium abscessus.
Richard, M., Gutierrez, A.V., Viljoen, A.J., Ghigo, E., Blaise, M., Kremer, L.(2018) Front Microbiol 9: 649-649
- PubMed: 29675007
- DOI: https://doi.org/10.3389/fmicb.2018.00649
- Primary Citation of Related Structures:
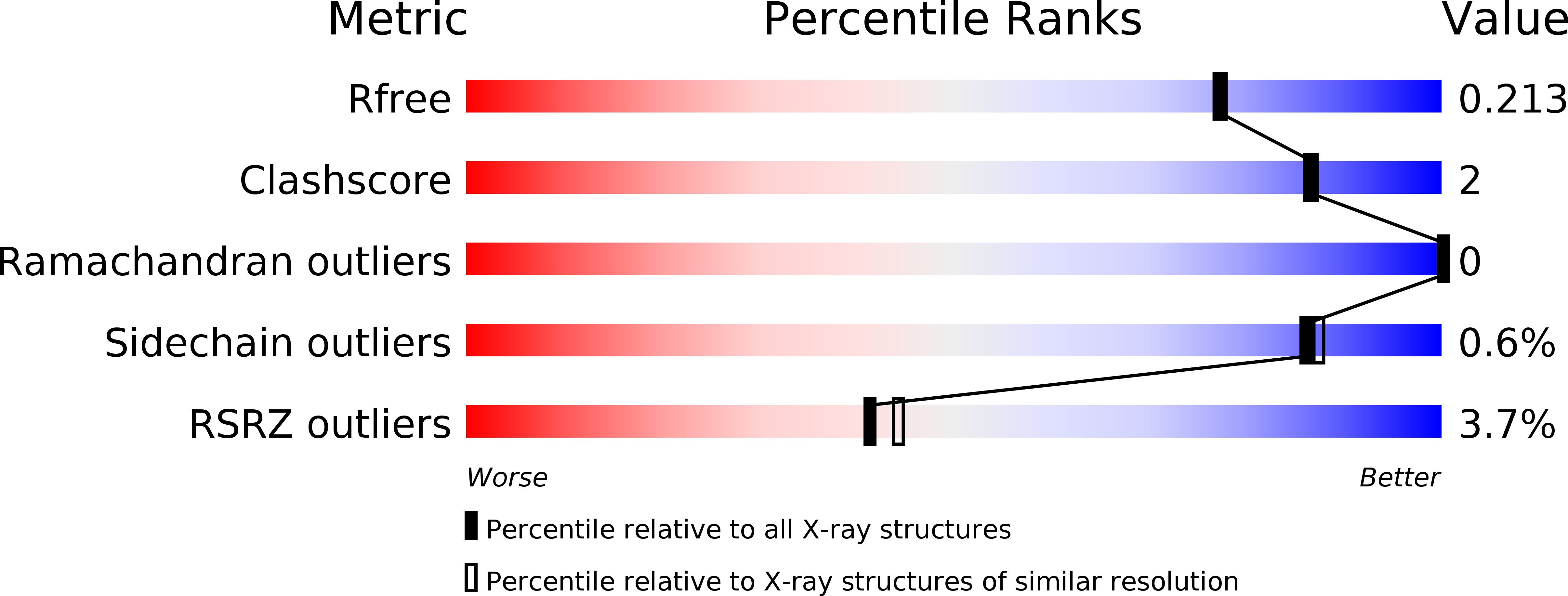
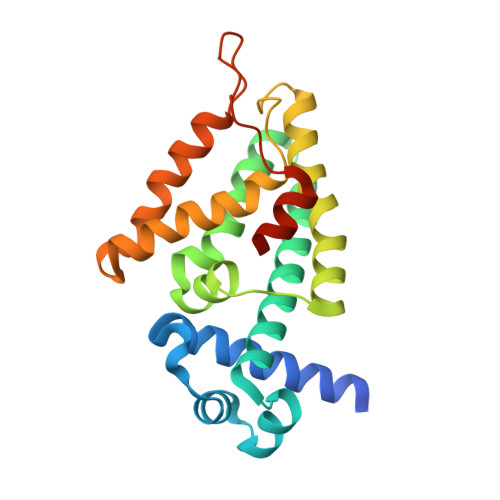
5OVY - PubMed Abstract:
Mycobacterium abscessus is an emerging human pathogen causing severe pulmonary infections and is refractory to standard antibiotherapy, yet few drug resistance mechanisms have been reported in this organism. Recently, mutations in MAB_4384 leading to up-regulation of the MmpS5/MmpL5 efflux pump were linked to increased resistance to thiacetazone derivatives. Herein, the DNA-binding activity of MAB_4384 was investigated by electrophoretic mobility shift assays using the palindromic sequence IR S5/L5 located upstream of mmpS5/mmpL5 . Introduction of point mutations within IR S5/L5 identified the sequence requirements for optimal binding of the regulator. Moreover, formation of the protein/IR S5/L5 complex was severely impaired for MAB_4384 harboring D14N or F57L substitutions. IR S5/L5 /lacZ reporter fusions in M. abscessus demonstrated increased β-galactosidase activity either in strains lacking a functional MAB_4384 or in cultures treated with the TAC analogs. In addition, X-ray crystallography confirmed a typical TetR homodimeric structure of MAB_4384 and unraveled a putative ligand binding site in which the analogs could be docked. Overall, these results support drug recognition of the MAB_4384 TetR regulator, alleviating its binding to IR S5/L5 and steering up-regulation of MmpS5/MmpL5. This study provides new mechanistic and structural details of TetR-dependent regulatory mechanisms of efflux pumps and drug resistance in mycobacteria.
Organizational Affiliation:
CNRS UMR 9004, Institut de Recherche en Infectiologie de Montpellier, Université de Montpellier, Montpellier, France.