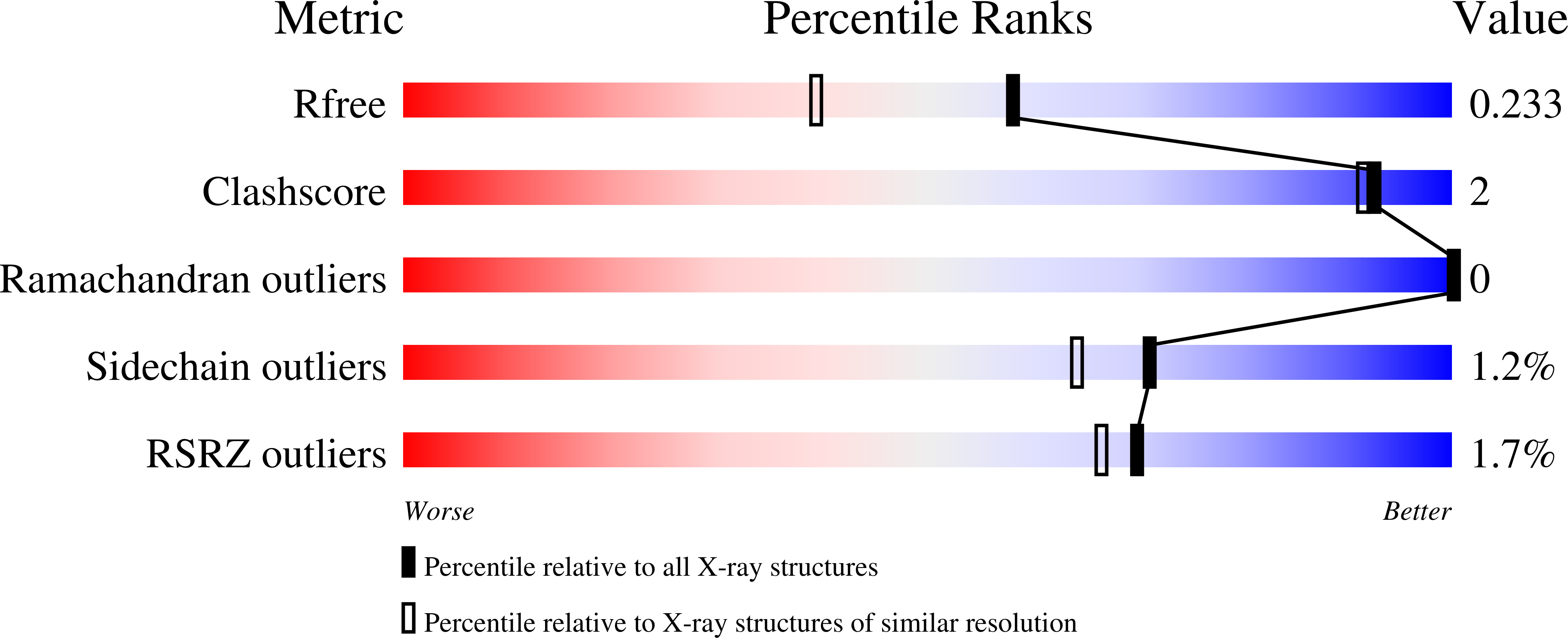
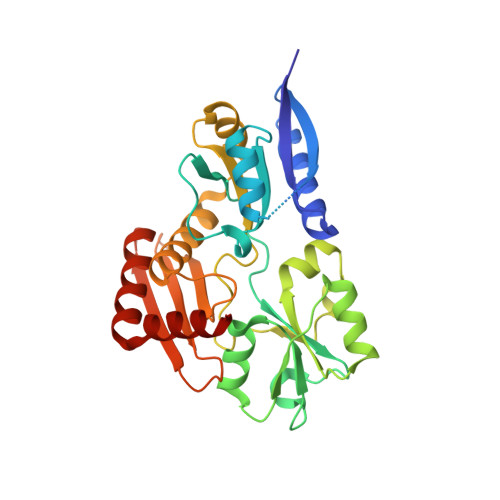
Structural studies of domain movement in active-site mutants of porphobilinogen deaminase from Bacillus megaterium.
Guo, J., Erskine, P., Coker, A.R., Wood, S.P., Cooper, J.B.(2017) Acta Crystallogr F Struct Biol Commun 73: 612-620
- PubMed: 29095155
- DOI: https://doi.org/10.1107/S2053230X17015436
- Primary Citation of Related Structures:
5OV4, 5OV5, 5OV6 - PubMed Abstract:
The enzyme porphobilinogen deaminase (PBGD) is one of the key enzymes in tetrapyrrole biosynthesis. It catalyses the formation of a linear tetrapyrrole from four molecules of the substrate porphobilinogen (PBG). It has a dipyrromethane cofactor (DPM) in the active site which is covalently linked to a conserved cysteine residue through a thioether bridge. The substrate molecules are linked to the cofactor in a stepwise head-to-tail manner during the reaction, which is catalysed by a conserved aspartate residue: Asp82 in the B. megaterium enzyme. Three mutations have been made affecting Asp82 (D82A, D82E and D82N) and their crystal structures have been determined at resolutions of 2.7, 1.8 and 1.9 Å, respectively. These structures reveal that whilst the D82E mutant possesses the DPM cofactor, in the D82N and D82A mutants the cofactor is likely to be missing, incompletely assembled or disordered. Comparison of the mutant PBGD structures with that of the wild-type enzyme shows that there are significant domain movements and suggests that the enzyme adopts `open' and `closed' conformations, potentially in response to substrate binding.
Organizational Affiliation:
Division of Medicine, University College London, Gower Street, London WC1E 6BT, England.