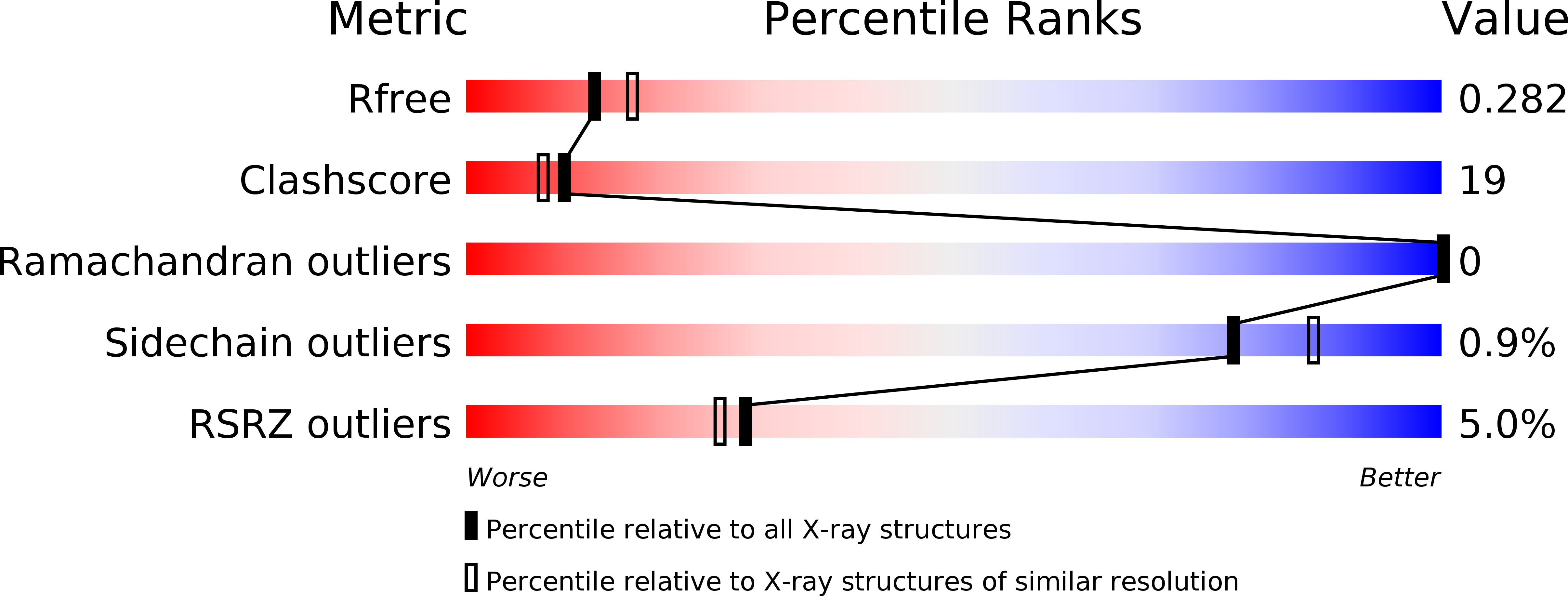
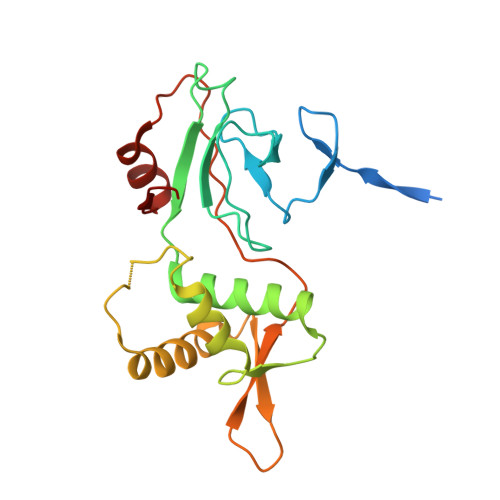
DNA binding by PHF1 prolongs PRC2 residence time on chromatin and thereby promotes H3K27 methylation.
Choi, J., Bachmann, A.L., Tauscher, K., Benda, C., Fierz, B., Muller, J.(2017) Nat Struct Mol Biol 24: 1039-1047
- PubMed: 29058710
- DOI: https://doi.org/10.1038/nsmb.3488
- Primary Citation of Related Structures:
5OQD - PubMed Abstract:
Polycomb repressive complex 2 (PRC2) trimethylates histone H3 at lysine 27 to mark genes for repression. We measured the dynamics of PRC2 binding on recombinant chromatin and free DNA at the single-molecule level using total internal reflection fluorescence (TIRF) microscopy. PRC2 preferentially binds free DNA with multisecond residence time and midnanomolar affinity. PHF1, a PRC2 accessory protein of the Polycomblike family, extends PRC2 residence time on DNA and chromatin. Crystallographic and functional studies reveal that Polycomblike proteins contain a winged-helix domain that binds DNA in a sequence-nonspecific fashion. DNA binding by this winged-helix domain accounts for the prolonged residence time of PHF1-PRC2 on chromatin and makes it a more efficient H3K27 methyltranferase than PRC2 alone. Together, these studies establish that interactions with DNA provide the predominant binding affinity of PRC2 for chromatin. Moreover, they reveal the molecular basis for how Polycomblike proteins stabilize PRC2 on chromatin and stimulate its activity.
Organizational Affiliation:
Max Planck Institute of Biochemistry, Laboratory of Chromatin Biology, Martinsried, Germany.