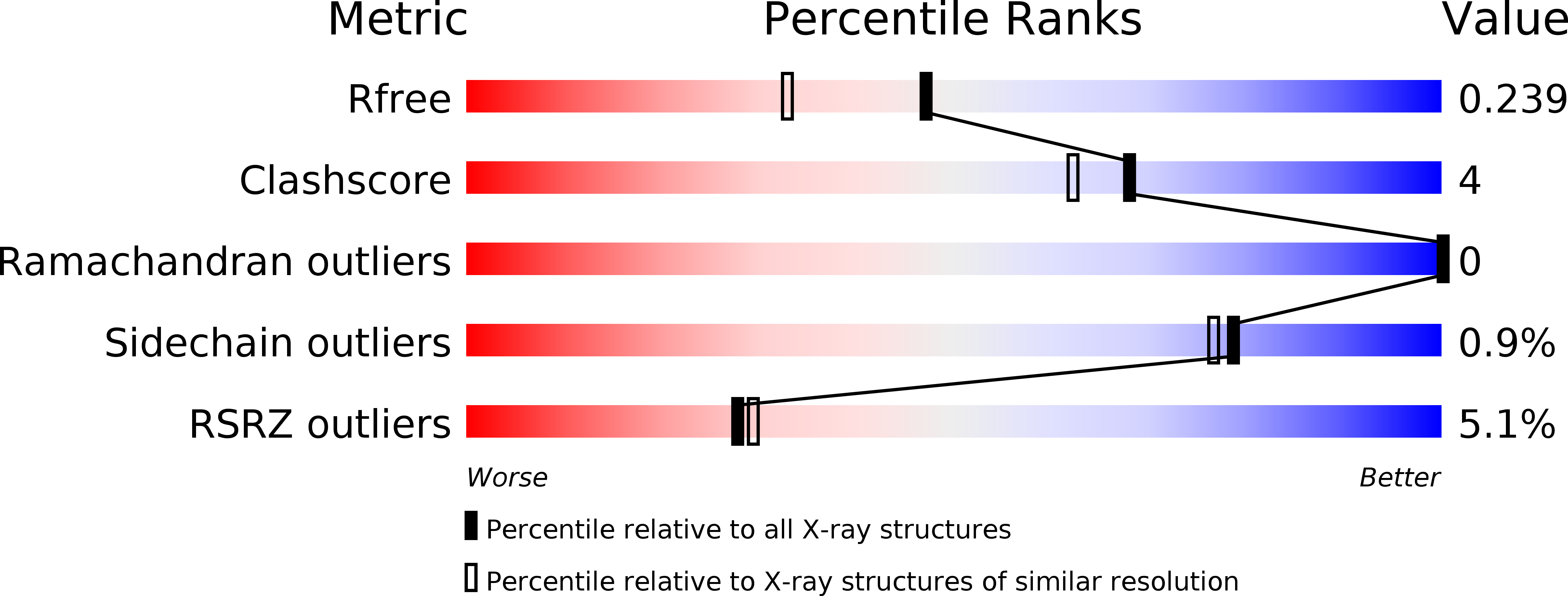
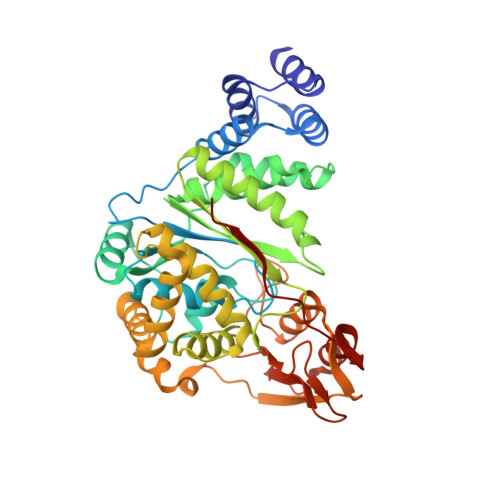
Crystal structure of pyrimidine-nucleoside phosphorylase from Bacillus subtilis in complex with imidazole and sulfate.
Balaev, V.V., Prokofev, I.I., Gabdoulkhakov, A.G., Betzel, C., Lashkov, A.A.(2018) Acta Crystallogr F Struct Biol Commun 74: 193-197
- PubMed: 29633966
- DOI: https://doi.org/10.1107/S2053230X18002935
- Primary Citation of Related Structures:
5OLN - PubMed Abstract:
Pyrimidine-nucleoside phosphorylase catalyzes the phosphorolytic cleavage of thymidine and uridine with equal activity. Investigation of this protein is essential for anticancer drug design. Here, the structure of this protein from Bacillus subtilis in complex with imidazole and sulfate is reported at 1.9 Å resolution, which is an improvement on the previously reported structure at 2.6 Å resolution. The localization and position of imidazole in the nucleoside-binding site reflects the possible binding of ligands that possess an imidazole ring.
Organizational Affiliation:
A. V. Shubnikov Institute of Crystallography, Leninsky Prospect 59, Moscow 119333, Russian Federation.