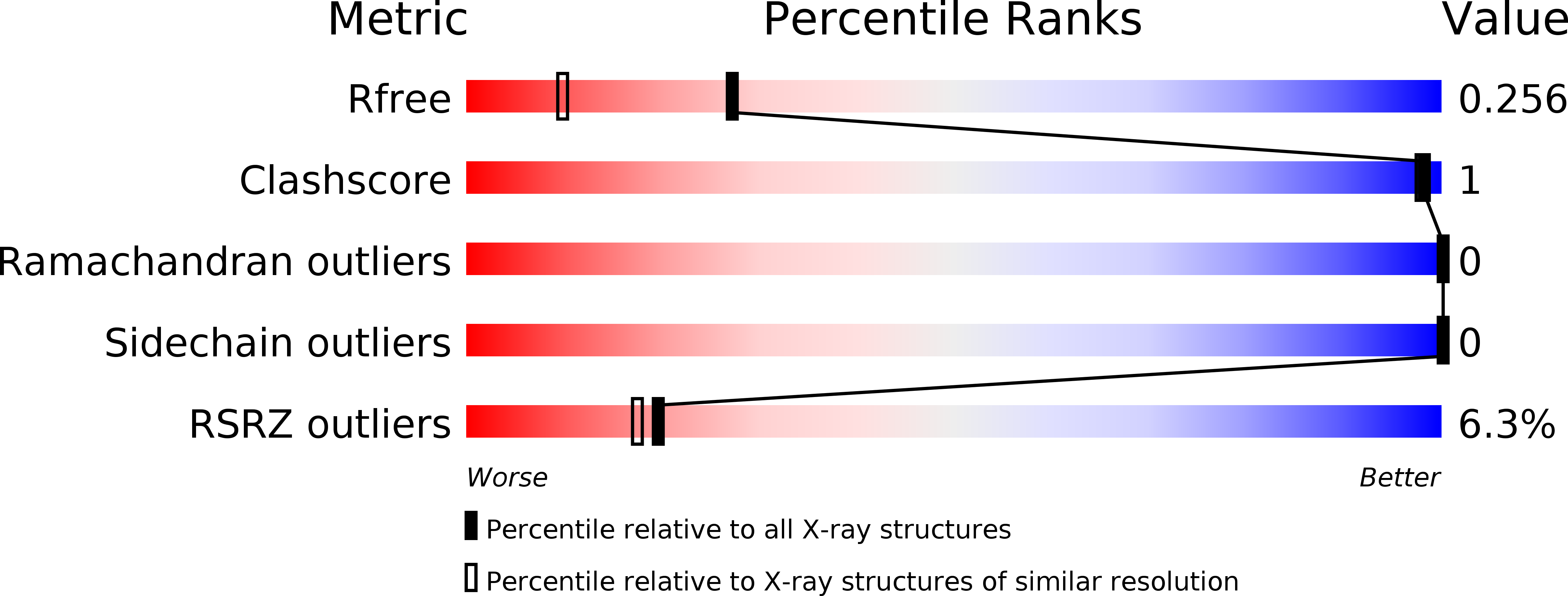
Enzyme catalysis captured using multiple structures from one crystal at varying temperatures.
Horrell, S., Kekilli, D., Sen, K., Owen, R.L., Dworkowski, F.S.N., Antonyuk, S.V., Keal, T.W., Yong, C.W., Eady, R.R., Hasnain, S.S., Strange, R.W., Hough, M.A.(2018) IUCrJ 5: 283-292
- PubMed: 29755744
- DOI: https://doi.org/10.1107/S205225251800386X
- Primary Citation of Related Structures:
5OF5, 5OF6, 5OF7, 5OF8, 5OFC, 5OFD, 5OFE, 5OFF, 5OFG, 5OFH, 5OG2, 5OG3, 5OG4, 5OG5, 5OG6, 5OGF, 5OGG - PubMed Abstract:
High-resolution crystal structures of enzymes in relevant redox states have transformed our understanding of enzyme catalysis. Recent developments have demonstrated that X-rays can be used, via the generation of solvated electrons, to drive reactions in crystals at cryogenic temperatures (100 K) to generate 'structural movies' of enzyme reactions. However, a serious limitation at these temperatures is that protein conformational motion can be significantly supressed. Here, the recently developed MSOX (multiple serial structures from one crystal) approach has been applied to nitrite-bound copper nitrite reductase at room temperature and at 190 K, close to the glass transition. During both series of multiple structures, nitrite was initially observed in a 'top-hat' geometry, which was rapidly transformed to a 'side-on' configuration before conversion to side-on NO, followed by dissociation of NO and substitution by water to reform the resting state. Density functional theory calculations indicate that the top-hat orientation corresponds to the oxidized type 2 copper site, while the side-on orientation is consistent with the reduced state. It is demonstrated that substrate-to-product conversion within the crystal occurs at a lower radiation dose at 190 K, allowing more of the enzyme catalytic cycle to be captured at high resolution than in the previous 100 K experiment. At room temperature the reaction was very rapid, but it remained possible to generate and characterize several structural states. These experiments open up the possibility of obtaining MSOX structural movies at multiple temperatures (MSOX-VT), providing an unparallelled level of structural information during catalysis for redox enzymes.
Organizational Affiliation:
School of Biological Sciences, University of Essex, Wivenhoe Park, Colchester CO4 3SQ, England.