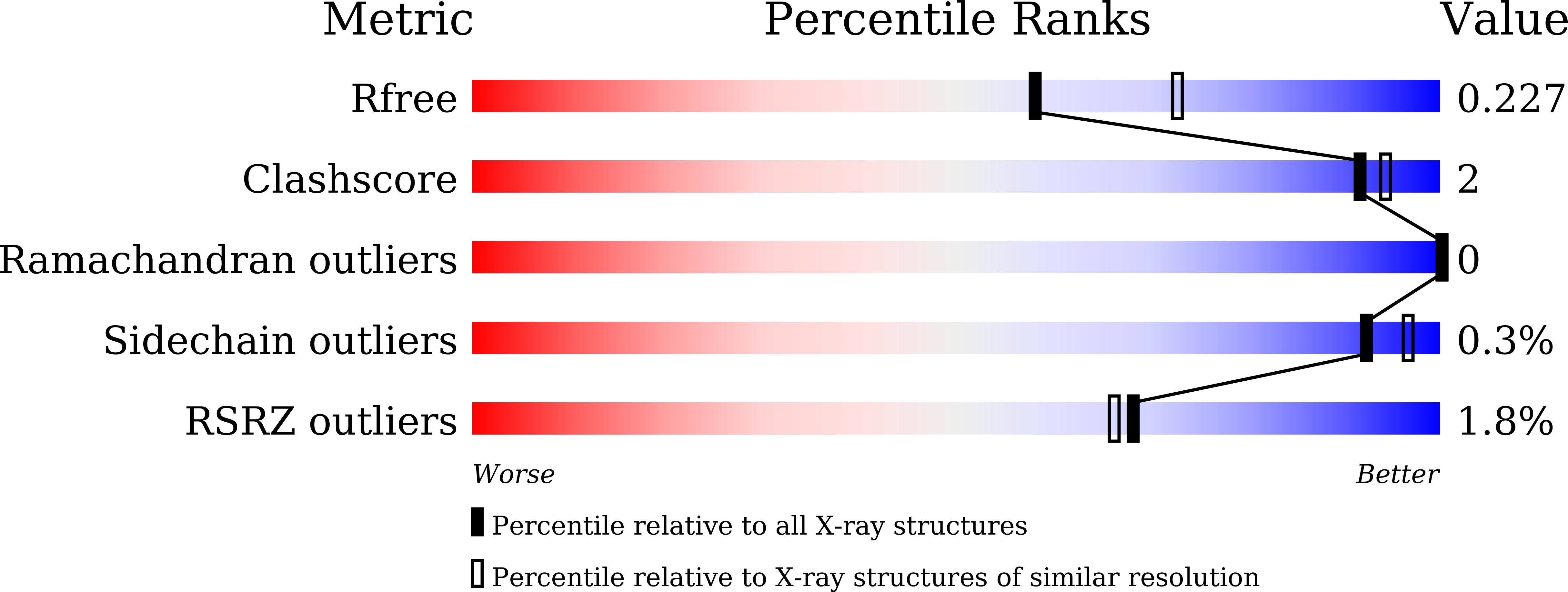
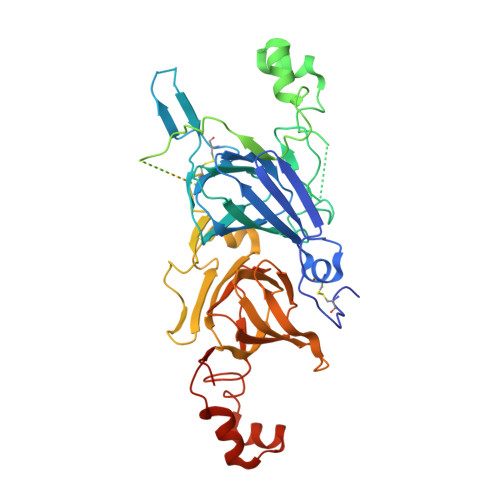
Crystallization and crystallographic studies of a novel chickpea 11S globulin.
Sun, L., Zhou, A., Zhang, F.(2022) Acta Crystallogr F Struct Biol Commun 78: 324-329
- PubMed: 36048082
- DOI: https://doi.org/10.1107/S2053230X22007919
- Primary Citation of Related Structures:
5GYL - PubMed Abstract:
Chickpea is a crop that is known as a source of high-quality proteins. CL-AI, which belongs to the 11S globulin and cupin superfamily, was initially identified in chickpea seeds. CL-AI has recently been shown to inhibit various types of α-amylases. To determine its molecular mechanism, the crystal structure of CL-AI was solved at a final resolution of 2.2 Å. Structural analysis indicated that each asymmetric unit contains three molecules with threefold symmetry and a head-to-tail association, and each molecule is divided into an α-chain and a β-chain. CL-AI has high structural similarity to other 11S globulins and canonical metal-dependent enzyme-related cupin proteins, whereas its stimilarity to α-amylase inhibitor from Phaseolus vulgaris is quite low. The structure presented here will provide insight into the function of CL-AI.
Organizational Affiliation:
Department of Dermatology, People's Hospital of SND, Suzhou, Jiangsu 215129, People's Republic of China.