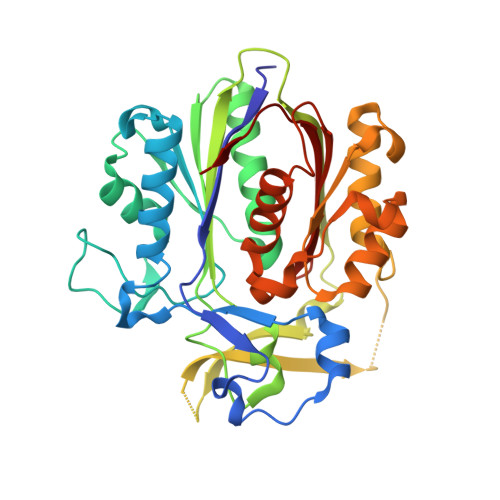
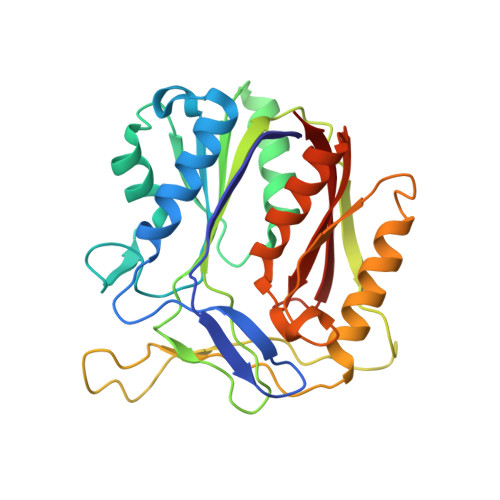
PqsBC, a Condensing Enzyme in the Biosynthesis of the Pseudomonas aeruginosa Quinolone Signal: CRYSTAL STRUCTURE, INHIBITION, AND REACTION MECHANISM.
Drees, S.L., Li, C., Prasetya, F., Saleem, M., Dreveny, I., Williams, P., Hennecke, U., Emsley, J., Fetzner, S.(2016) J Biol Chem 291: 6610-6624
- PubMed: 26811339
- DOI: https://doi.org/10.1074/jbc.M115.708453
- Primary Citation of Related Structures:
5DWZ - PubMed Abstract:
Pseudomonas aeruginosaproduces a number of alkylquinolone-type secondary metabolites best known for their antimicrobial effects and involvement in cell-cell communication. In the alkylquinolone biosynthetic pathway, the β-ketoacyl-(acyl carrier protein) synthase III (FabH)-like enzyme PqsBC catalyzes the condensation of octanoyl-coenzyme A and 2-aminobenzoylacetate (2-ABA) to form the signal molecule 2-heptyl-4(1H)-quinolone. PqsBC, a potential drug target, is unique for its heterodimeric arrangement and an active site different from that of canonical FabH-like enzymes. Considering the sequence dissimilarity between the subunits, a key question was how the two subunits are organized with respect to the active site. In this study, the PqsBC structure was determined to a 2 Å resolution, revealing that PqsB and PqsC have a pseudo-2-fold symmetry that unexpectedly mimics the FabH homodimer. PqsC has an active site composed of Cys-129 and His-269, and the surrounding active site cleft is hydrophobic in character and approximately twice the volume of related FabH enzymes that may be a requirement to accommodate the aromatic substrate 2-ABA. From physiological and kinetic studies, we identified 2-aminoacetophenone as a pathway-inherent competitive inhibitor of PqsBC, whose fluorescence properties could be used forin vitrobinding studies. In a time-resolved setup, we demonstrated that the catalytic histidine is not involved in acyl-enzyme formation, but contributes to an acylation-dependent increase in affinity for the second substrate 2-ABA. Introduction of Asn into the PqsC active site led to significant activity toward the desamino substrate analog benzoylacetate, suggesting that the substrate 2-ABA itself supplies the asparagine-equivalent amino function that assists in catalysis.
Organizational Affiliation:
From the Institute for Molecular Microbiology and Biotechnology and.