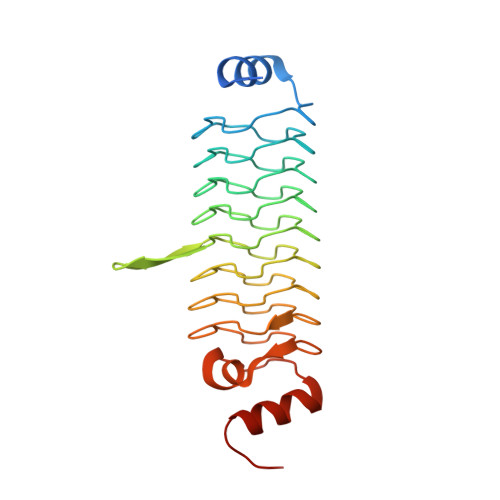
Synthetic beta-solenoid proteins with the fragment-free computational design of a beta-hairpin extension.
MacDonald, J.T., Kabasakal, B.V., Godding, D., Kraatz, S., Henderson, L., Barber, J., Freemont, P.S., Murray, J.W.(2016) Proc Natl Acad Sci U S A 113: 10346-10351
- PubMed: 27573845
- DOI: https://doi.org/10.1073/pnas.1525308113
- Primary Citation of Related Structures:
4YC5, 4YCQ, 4YDT, 4YEI, 4YFO, 5DI5, 5DN0, 5DNS, 5DQA, 5DRA, 5DZB - PubMed Abstract:
The ability to design and construct structures with atomic level precision is one of the key goals of nanotechnology. Proteins offer an attractive target for atomic design because they can be synthesized chemically or biologically and can self-assemble. However, the generalized protein folding and design problem is unsolved. One approach to simplifying the problem is to use a repetitive protein as a scaffold. Repeat proteins are intrinsically modular, and their folding and structures are better understood than large globular domains. Here, we have developed a class of synthetic repeat proteins based on the pentapeptide repeat family of beta-solenoid proteins. We have constructed length variants of the basic scaffold and computationally designed de novo loops projecting from the scaffold core. The experimentally solved 3.56-Å resolution crystal structure of one designed loop matches closely the designed hairpin structure, showing the computational design of a backbone extension onto a synthetic protein core without the use of backbone fragments from known structures. Two other loop designs were not clearly resolved in the crystal structures, and one loop appeared to be in an incorrect conformation. We have also shown that the repeat unit can accommodate whole-domain insertions by inserting a domain into one of the designed loops.
Organizational Affiliation:
Centre for Synthetic Biology and Innovation, Imperial College London, London SW7 2AZ, United Kingdom; Department of Medicine, Imperial College London, London SW7 2AZ, United Kingdom;