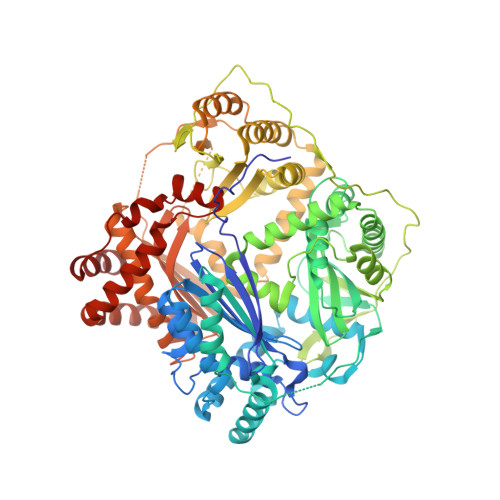
Crystal Structure and Function of PqqF Protein in the Pyrroloquinoline Quinone Biosynthetic Pathway
Wei, Q., Ran, T., Ma, C., He, J., Xu, D., Wang, W.(2016) J Biol Chem 291: 15575-15587
- PubMed: 27231346
- DOI: https://doi.org/10.1074/jbc.M115.711226
- Primary Citation of Related Structures:
5CIO - PubMed Abstract:
Pyrroloquinoline quinone (PQQ) has received considerable attention due to its numerous important physiological functions. PqqA is a precursor peptide of PQQ with two conserved residues: glutamate and tyrosine. After linkage of the Cγ of glutamate and Cϵ of tyrosine by PqqE, these two residues are hypothesized to be cleaved from PqqA by PqqF. The linked glutamate and tyrosine residues are then used to synthesize PQQ. Here, we demonstrated that the pqqF gene is essential for PQQ biosynthesis as deletion of it eliminated the inhibition of prodigiosin production by glucose. We further determined the crystal structure of PqqF, which has a closed clamshell-like shape. The PqqF consists of two halves composed of an N- and a C-terminal lobe. The PqqF-N and PqqF-C lobes form a chamber with the volume of the cavity of ∼9400 Å(3) The PqqF structure conforms to the general structure of inverzincins. Compared with the most thoroughly characterized inverzincin insulin-degrading enzyme, the size of PqqF chamber is markedly smaller, which may define the specificity for its substrate PqqA. Furthermore, the 14-amino acid-residue-long tag formed by the N-terminal tag from expression vector precisely protrudes into the counterpart active site; this N-terminal tag occupies the active site and stabilizes the closed, inactive conformation. His-48, His-52, Glu-129 and His-14 from the N-terminal tag coordinate with the zinc ion. Glu-51 acts as a base catalyst. The observed histidine residue-mediated inhibition may be applicable for the design of a peptide for the inhibition of M16 metalloproteases.
Organizational Affiliation:
From the Department of microbiology, College of Life Sciences, Nanjing Agricultural University, 210095 Nanjing, China and.