Crystal structures of nematode (parasitic T. spiralis and free living C. elegans), compared to mammalian, thymidylate synthases (TS). Molecular docking and molecular dynamics simulations in search for nematode-specific inhibitors of TS.
Jarmula, A., Wilk, P., Maj, P., Ludwiczak, J., Dowiercial, A., Banaszak, K., Rypniewski, W., Ciesla, J., Dabrowska, M., Fraczyk, T., Bronowska, A.K., Jakowiecki, J., Filipek, S., Rode, W.(2017) J Mol Graph Model 77: 33-50
- PubMed: 28826032
- DOI: https://doi.org/10.1016/j.jmgm.2017.08.008
- Primary Citation of Related Structures:
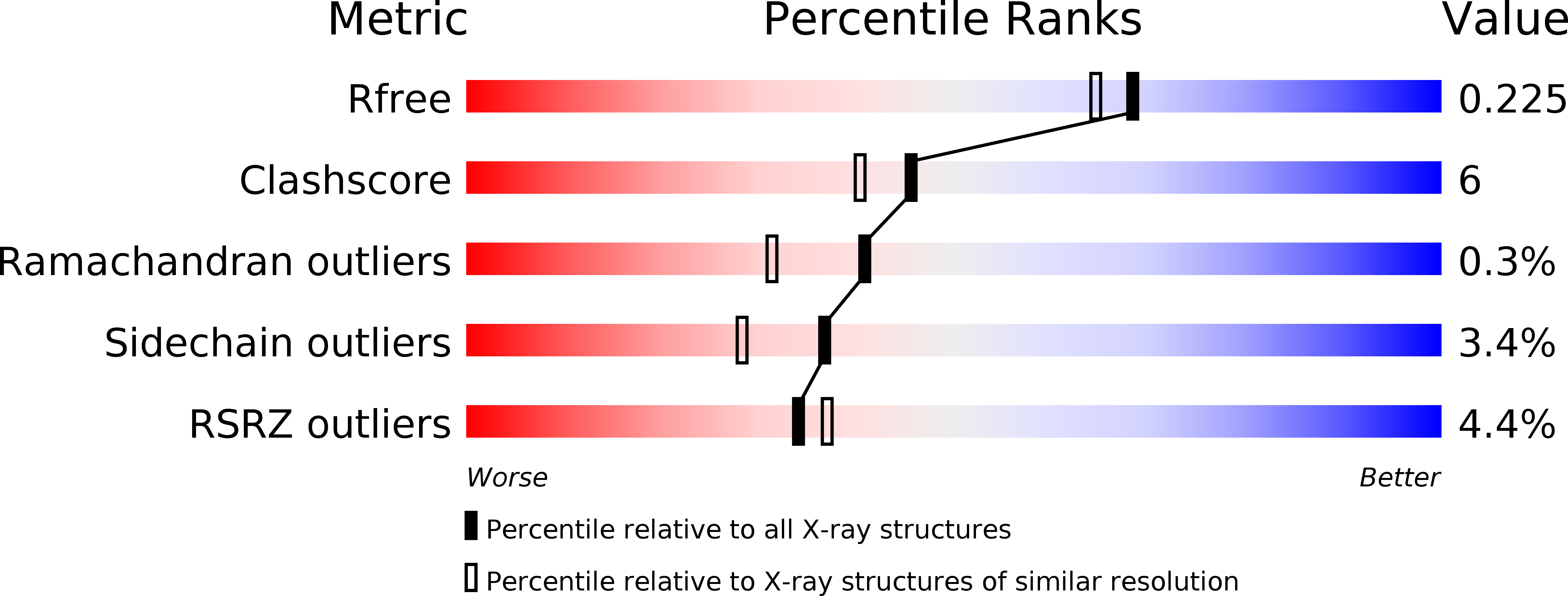
4IQB, 5BY6, 5NOO - PubMed Abstract:
Three crystal structures are presented of nematode thymidylate synthases (TS), including Caenorhabditis elegans (Ce) enzyme without ligands and its ternary complex with dUMP and Raltitrexed, and binary complex of Trichinella spiralis (Ts) enzyme with dUMP. In search of differences potentially relevant for the development of species-specific inhibitors of the nematode enzyme, a comparison was made of the present Ce and Ts enzyme structures, as well as binary complex of Ce enzyme with dUMP, with the corresponding mammalian (human, mouse and rat) enzyme crystal structures. To complement the comparison, tCONCOORD computations were performed to evaluate dynamic behaviors of mammalian and nematode TS structures. Finally, comparative molecular docking combined with molecular dynamics and free energy of binding calculations were carried out to search for ligands showing selective affinity to T. spiralis TS. Despite an overall strong similarity in structure and dynamics of nematode vs mammalian TSs, a pool of ligands demonstrating predictively a strong and selective binding to TsTS has been delimited. These compounds, the E63 family, locate in the dimerization interface of TsTS where they exert species-specific interactions with certain non-conserved residues, including hydrogen bonds with Thr174 and hydrophobic contacts with Phe192, Cys191 and Tyr152. The E63 family of ligands opens the possibility of future development of selective inhibitors of TsTS and effective agents against trichinellosis.
Organizational Affiliation:
Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warszawa, Poland. Electronic address: a.jarmula@nencki.gov.pl.