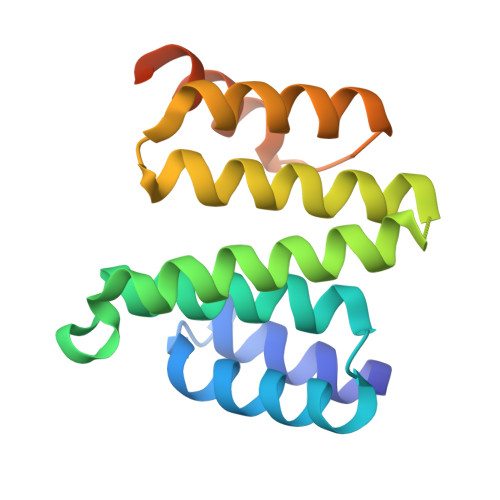
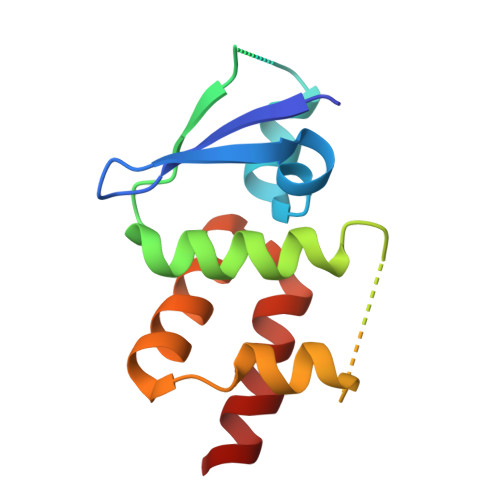
The crystal structure of the Sgt1-Skp1 complex: the link between Hsp90 and both SCF E3 ubiquitin ligases and kinetochores.
Willhoft, O., Kerr, R., Patel, D., Zhang, W., Al-Jassar, C., Daviter, T., Millson, S.H., Thalassinos, K., Vaughan, C.K.(2017) Sci Rep 7: 41626-41626
- PubMed: 28139700
- DOI: https://doi.org/10.1038/srep41626
- Primary Citation of Related Structures:
5AN3 - PubMed Abstract:
The essential cochaperone Sgt1 recruits Hsp90 chaperone activity to a range of cellular factors including SCF E3 ubiquitin ligases and the kinetochore in eukaryotes. In these pathways Sgt1 interacts with Skp1, a small protein that heterodimerizes with proteins containing the F-box motif. We have determined the crystal structure of the interacting domains of Saccharomyces cerevisiae Sgt1 and Skp1 at 2.8 Å resolution and validated the interface in the context of the full-length proteins in solution. The BTB/POZ domain of Skp1 associates with Sgt1 via the concave surface of its TPR domain using residues that are conserved in humans. Dimerization of yeast Sgt1 occurs via an insertion that is absent from monomeric human Sgt1. We identify point mutations that disrupt dimerization and Skp1 binding in vitro and find that the interaction with Skp1 is an essential function of Sgt1 in yeast. Our data provide a structural rationale for understanding the phenotypes of temperature-sensitive Sgt1 mutants and for linking Skp1-associated proteins to Hsp90-dependent pathways.
Organizational Affiliation:
Institute of Structural and Molecular Biology, University College London and Birkbeck, Biological Sciences, Malet Street, London, WC1E 7HX, UK.