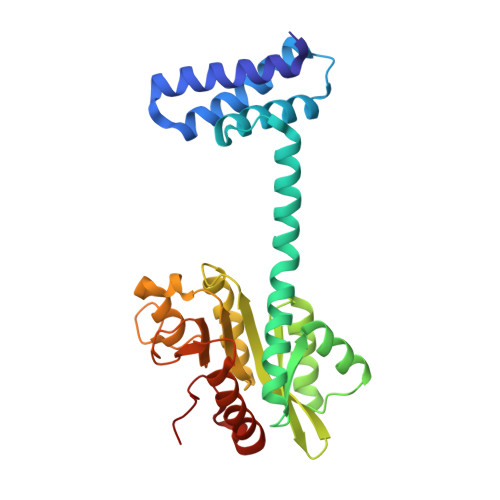
Functional significance of protein assemblies predicted by the crystal structure of the restriction endonuclease BsaWI.
Tamulaitis, G., Rutkauskas, M., Zaremba, M., Grazulis, S., Tamulaitiene, G., Siksnys, V.(2015) Nucleic Acids Res 43: 8100-8110
- PubMed: 26240380
- DOI: https://doi.org/10.1093/nar/gkv768
- Primary Citation of Related Structures:
4ZSF - PubMed Abstract:
Type II restriction endonuclease BsaWI recognizes a degenerated sequence 5'-W/CCGGW-3' (W stands for A or T, '/' denotes the cleavage site). It belongs to a large family of restriction enzymes that contain a conserved CCGG tetranucleotide in their target sites. These enzymes are arranged as dimers or tetramers, and require binding of one, two or three DNA targets for their optimal catalytic activity. Here, we present a crystal structure and biochemical characterization of the restriction endonuclease BsaWI. BsaWI is arranged as an 'open' configuration dimer and binds a single DNA copy through a minor groove contacts. In the crystal primary BsaWI dimers form an indefinite linear chain via the C-terminal domain contacts implying possible higher order aggregates. We show that in solution BsaWI protein exists in a dimer-tetramer-oligomer equilibrium, but in the presence of specific DNA forms a tetramer bound to two target sites. Site-directed mutagenesis and kinetic experiments show that BsaWI is active as a tetramer and requires two target sites for optimal activity. We propose BsaWI mechanism that shares common features both with dimeric Ecl18kI/SgrAI and bona fide tetrameric NgoMIV/SfiI enzymes.
Organizational Affiliation:
Department of Protein-DNA Interactions, Institute of Biotechnology, Vilnius University, Graiciuno 8, LT-02241 Vilnius, Lithuania.