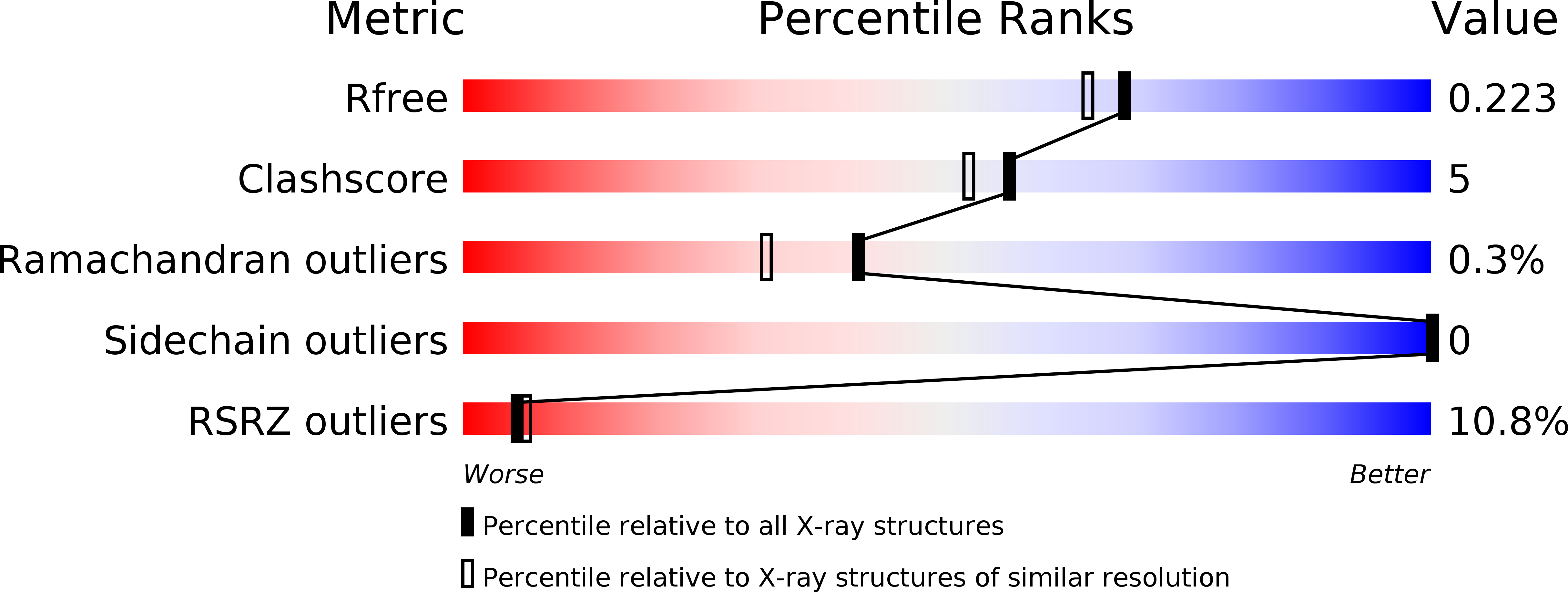
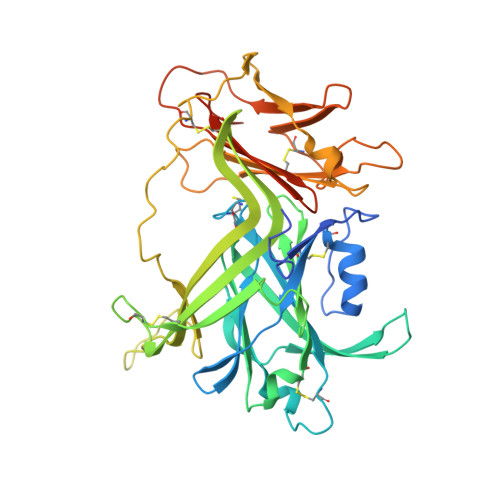
Parallel Evolution of Chemokine Binding by Structurally Related Herpesvirus Decoy Receptors.
Lubman, O.Y., Fremont, D.H.(2016) Structure 24: 57-69
- PubMed: 26671708
- DOI: https://doi.org/10.1016/j.str.2015.10.018
- Primary Citation of Related Structures:
4ZKQ, 4ZLT - PubMed Abstract:
A wide variety of pathogens targets chemokine signaling networks in order to disrupt host immune surveillance and defense. Here, we report a structural and mutational analysis of rodent herpesvirus Peru encoded R17, a potent chemokine inhibitor that sequesters CC and C chemokines with high affinity. R17 consists of a pair of β-sandwich domains linked together by a bridging sheet, which form an acidic binding cleft for the chemokine CCL3 on the opposite face of a basic surface cluster that binds glycosaminoglycans. R17 promiscuously engages chemokines primarily through the same N-loop determinants used for host receptor recognition while residues located in the chemokine 40s loop drive kinetically stable complex formation. The core fold adopted by R17 is unexpectedly similar to that of the M3 chemokine decoy receptor encoded by MHV-68, although, strikingly, neither the location of ligand engagement nor the stoichiometry of binding is conserved, suggesting that their functions evolved independently.
Organizational Affiliation:
Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO 63110, USA.