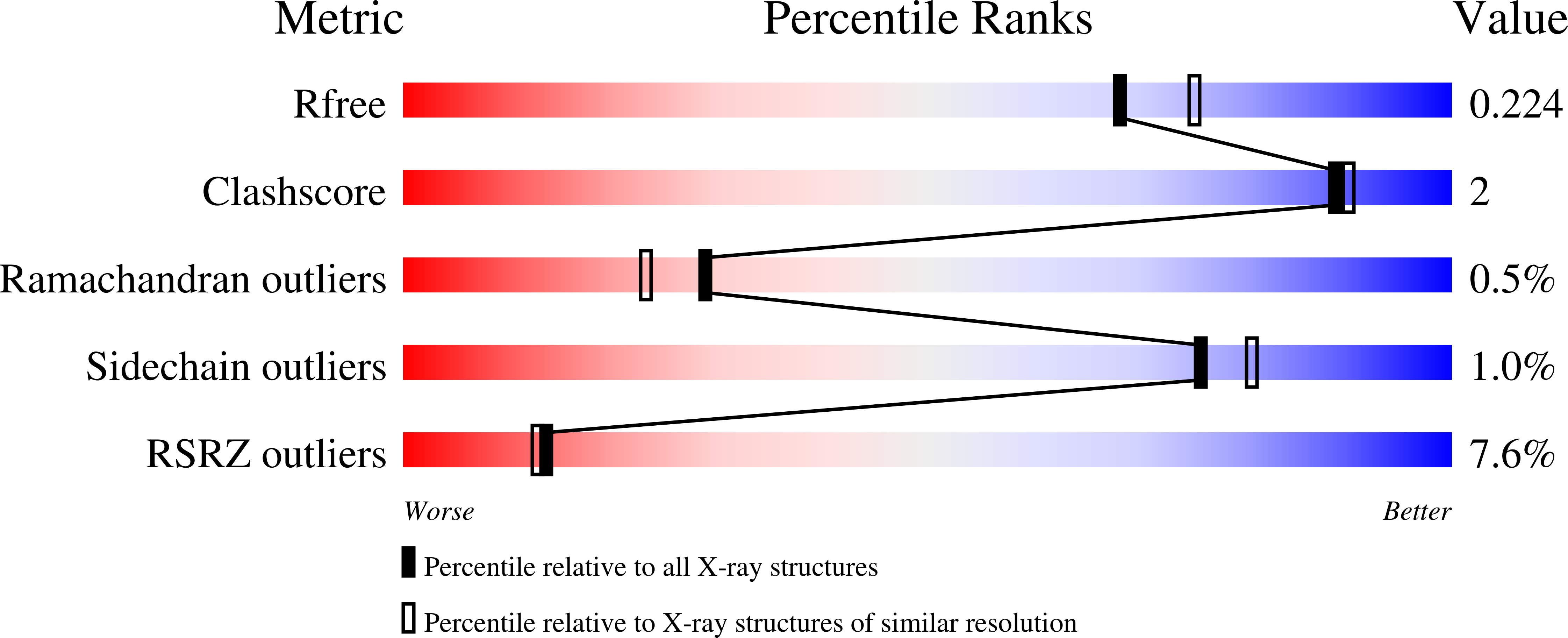
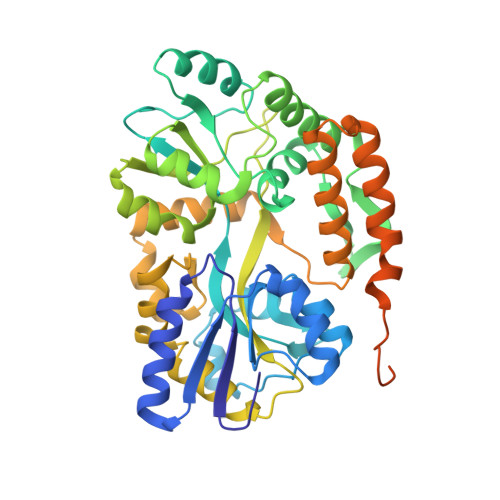
The 2.2-Angstrom resolution crystal structure of the carboxy-terminal region of ataxin-3.
Zhemkov, V.A., Kulminskaya, A.A., Bezprozvanny, I.B., Kim, M.(2016) FEBS Open Bio 6: 168-178
- PubMed: 27047745
- DOI: https://doi.org/10.1002/2211-5463.12029
- Primary Citation of Related Structures:
4WTH, 4YS9 - PubMed Abstract:
An expansion of polyglutamine (polyQ) sequence in ataxin-3 protein causes spinocerebellar ataxia type 3, an inherited neurodegenerative disorder. The crystal structure of the polyQ-containing carboxy-terminal fragment of human ataxin-3 was solved at 2.2-Å resolution. The Atxn3 carboxy-terminal fragment including 14 glutamine residues adopts both random coil and α-helical conformations in the crystal structure. The polyQ sequence in α-helical structure is stabilized by intrahelical hydrogen bonds mediated by glutamine side chains. The intrahelical hydrogen-bond interactions between glutamine side chains along the axis of the polyQ α-helix stabilize the secondary structure. Analysis of this structure furthers our understanding of the polyQ-structural characteristics that likely underlie the pathogenesis of polyQ-expansion disorders.
Organizational Affiliation:
Laboratory of Molecular Neurodegeneration St Petersburg State Polytechnical University Russia; Laboratory of Enzymology National Research Center «Kurchatov Institute»B.P. Konstantinov Petersburg Nuclear Physics Institute Gatchina Russia.