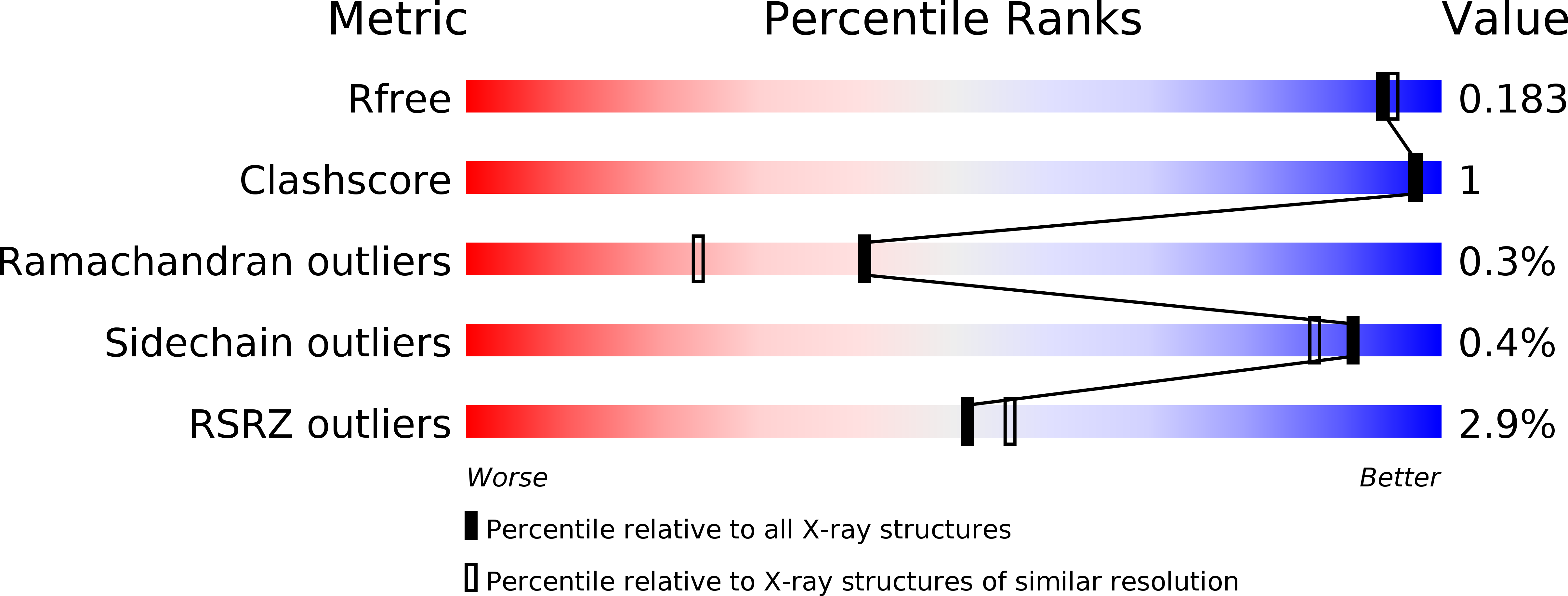
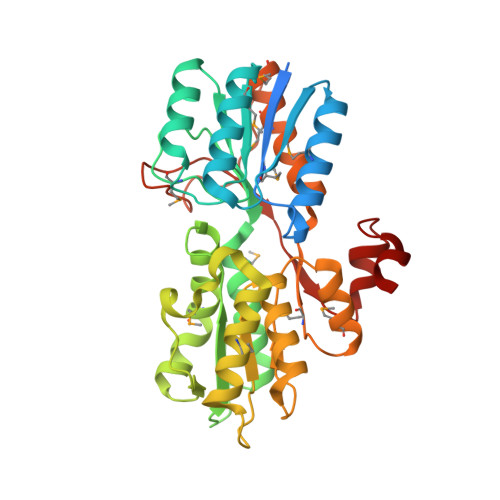
CRYSTAL STRUCTURE OF AN ABC TRANSPORTER SOLUTE BINDING PROTEIN (IPR025997) FROM CLOSTRIDIUM PHYTOFERMENTANS (Cphy_1585, TARGET EFI-511156) WITH BOUND BETA-D-GLUCOSE
Vetting, M.W., Patskovsky, Y., Al Obaidi, N.F., Toro, R., Morisco, L.L., Benach, J., Koss, J., Wasserman, S.R., Attonito, J.D., Scott Glenn, A., Chamala, S., Chowdhury, S., Lafleur, J., Love, J., Seidel, R.D., Whalen, K.L., Gerlt, J.A., Almo, S.C., Enzyme Function Initiative (EFI)To be published.