Discovery and characterization of natural tropolones as inhibitors of the antibacterial target CapF from Staphylococcus aureus.
Nakano, K., Chigira, T., Miyafusa, T., Nagatoishi, S., Caaveiro, J.M., Tsumoto, K.(2015) Sci Rep 5: 15337-15337
- PubMed: 26471247
- DOI: https://doi.org/10.1038/srep15337
- Primary Citation of Related Structures:
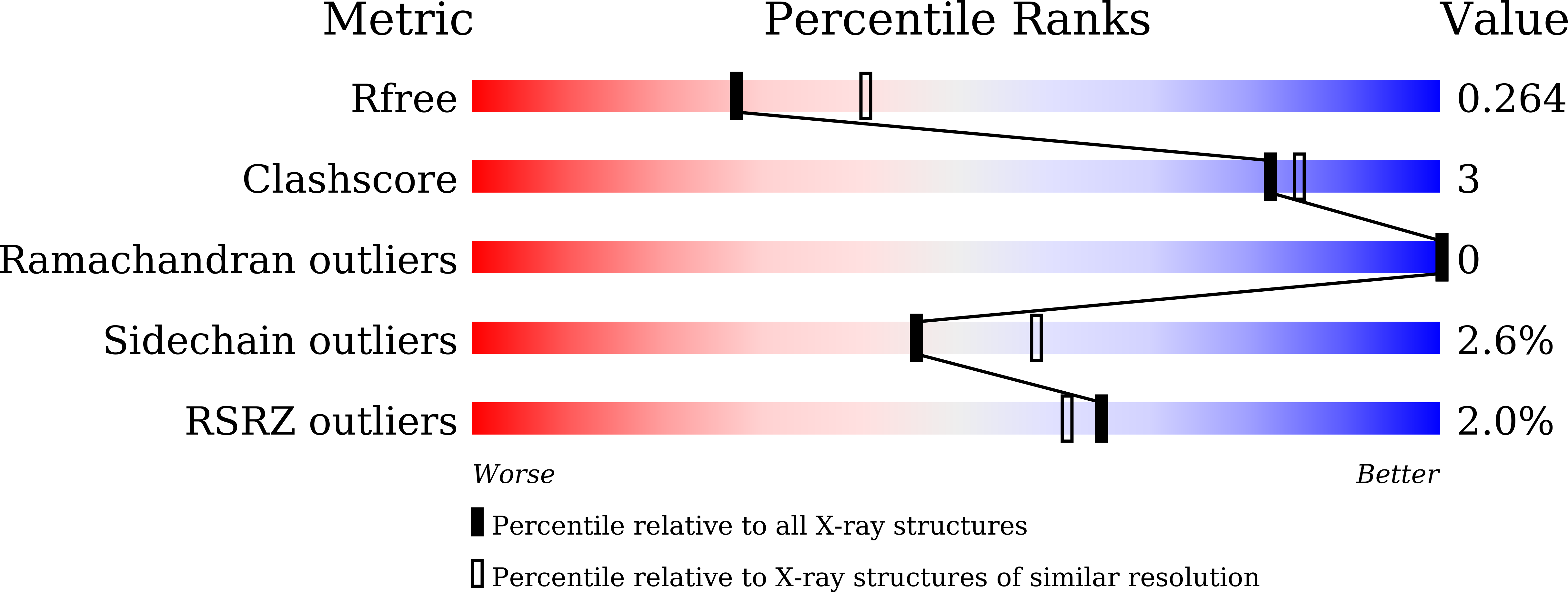
4YRD - PubMed Abstract:
The rapid spread of antibiotic-resistance among pathogenic bacteria poses a serious risk for public health. The search for novel therapeutic strategies and antimicrobial compounds is needed to ameliorate this menace. The bifunctional metalloenzyme CapF is an antibacterial target produced by certain pathogenic bacteria essential in the biosynthetic route of capsular polysaccharide, a mucous layer on the surface of bacterium that facilitates immune evasion and infection. We report the first inhibitor of CapF from Staphylococcus aureus, which was identified by employing fragment-based methodologies. The hit compound 3-isopropenyl-tropolone inhibits the first reaction catalyzed by CapF, disrupting the synthesis of a key precursor of capsular polysaccharide. Isothermal titration calorimetry demonstrates that 3-isopropenyl-tropolone binds tightly (KD = 27 ± 7 μM) to the cupin domain of CapF. In addition, the crystal structure of the enzyme-inhibitor complex shows that the compound engages the essential Zn(2+) ion necessary for the first reaction catalyzed by the enzyme, explaining its inhibitory effect. Moreover, the tropolone compound alters the coordination sphere of the metal, leading to the overall destabilization of the enzyme. We propose 3-isopropenyl-tropolone as a precursor to develop stronger inhibitors for this family of enzymes to impair the synthesis of capsular polysaccharide in Staphylococcus aureus.
Organizational Affiliation:
Department of Medical Genome Sciences, Graduate School of Frontier Sciences, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo 108-8639, Japan.