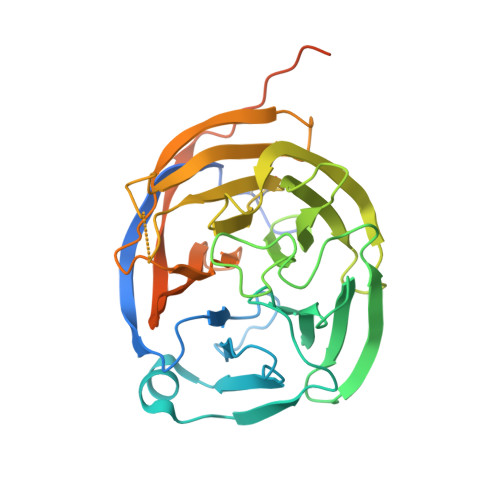
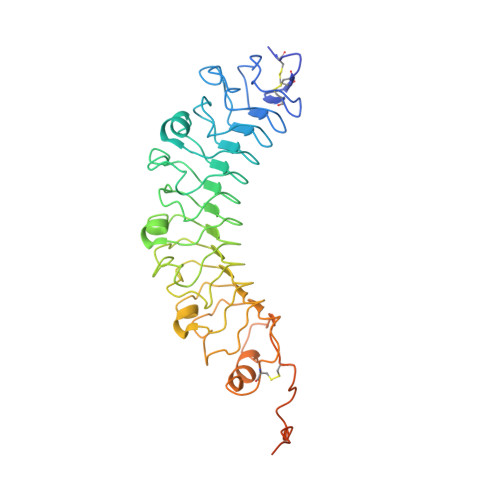
Structural and Mechanistic Insights into the Latrophilin3-FLRT3 Complex that Mediates Glutamatergic Synapse Development.
Ranaivoson, F.M., Liu, Q., Martini, F., Bergami, F., von Daake, S., Li, S., Lee, D., Demeler, B., Hendrickson, W.A., Comoletti, D.(2015) Structure 23: 1665-1677
- PubMed: 26235031
- DOI: https://doi.org/10.1016/j.str.2015.06.022
- Primary Citation of Related Structures:
4RMK, 4RML, 4YEB - PubMed Abstract:
Latrophilins (LPHNs) are adhesion-like G-protein-coupled receptors implicated in attention-deficit/hyperactivity disorder. Recently, LPHN3 was found to regulate excitatory synapse number through trans interactions with fibronectin leucine-rich repeat transmembrane 3 (FLRT3). By isothermal titration calorimetry, we determined that only the olfactomedin (OLF) domain of LPHN3 is necessary for FLRT3 association. By multi-crystal native single-wavelength anomalous diffraction phasing, we determined the crystal structure of the OLF domain. This structure is a five-bladed β propeller with a Ca(2+) ion bound in the central pore, which is capped by a mobile loop that allows the ion to exchange with the solvent. The crystal structure of the OLF/FLRT3 complex shows that LPHN3-OLF in the closed state binds with high affinity to the concave face of FLRT3-LRR with a combination of hydrophobic and charged residues. Our study provides structural and functional insights into the molecular mechanism underlying the contribution of LPHN3/FLRT3 to the development of glutamatergic synapses.
Organizational Affiliation:
Child Health Institute of New Jersey and Department of Neuroscience and Cell Biology, Robert Wood Johnson Medical School, Rutgers University, 89 French Street, New Brunswick, NJ 08901, USA.