Structural analysis of PseH, the Campylobacter jejuni N-acetyltransferase involved in bacterial O-linked glycosylation.
Song, W.S., Nam, M.S., Namgung, B., Yoon, S.I.(2015) Biochem Biophys Res Commun 458: 843-848
- PubMed: 25698400
- DOI: https://doi.org/10.1016/j.bbrc.2015.02.041
- Primary Citation of Related Structures:
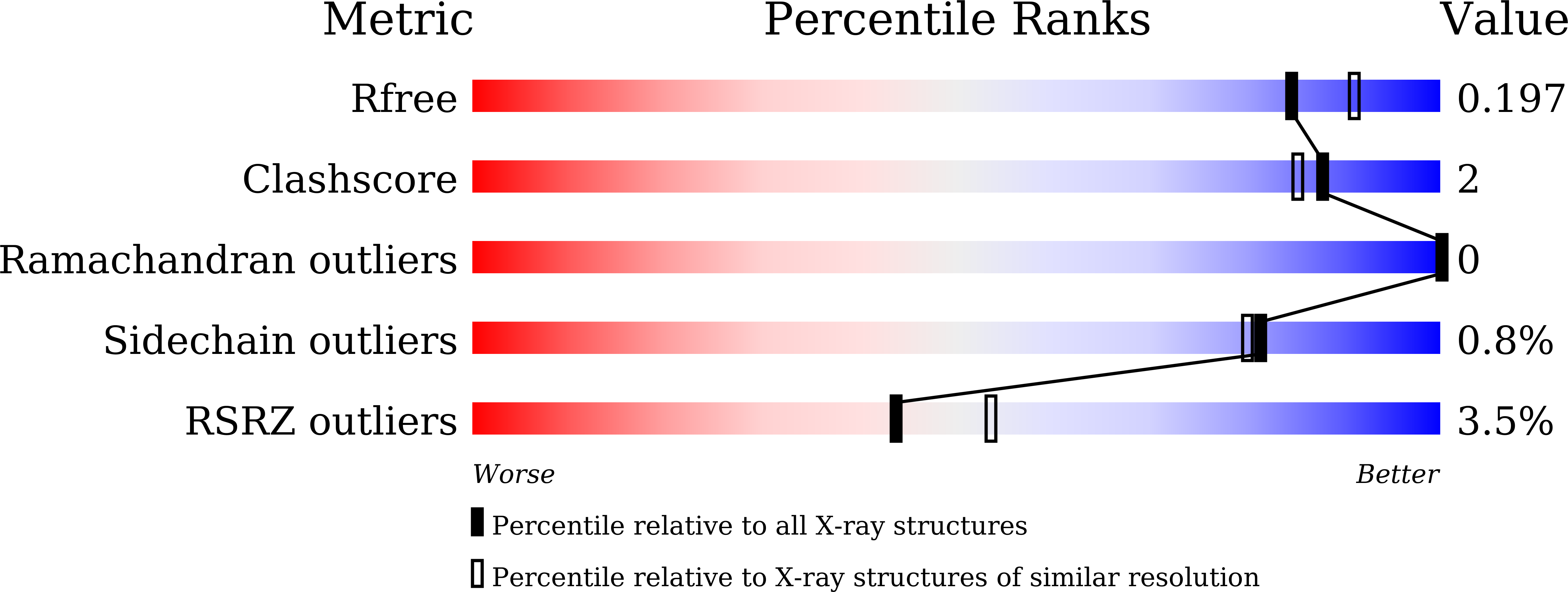
4XPK, 4XPL - PubMed Abstract:
Campylobacter jejuni is a bacterium that uses flagella for motility and causes worldwide acute gastroenteritis in humans. The C. jejuni N-acetyltransferase PseH (cjPseH) is responsible for the third step in flagellin O-linked glycosylation and plays a key role in flagellar formation and motility. cjPseH transfers an acetyl group from an acetyl donor, acetyl coenzyme A (AcCoA), to the amino group of UDP-4-amino-4,6-dideoxy-N-acetyl-β-L-altrosamine to produce UDP-2,4-diacetamido-2,4,6-trideoxy-β-L-altropyranose. To elucidate the catalytic mechanism of cjPseH, crystal structures of cjPseH alone and in complex with AcCoA were determined at 1.95 Å resolution. cjPseH folds into a single-domain structure of a central β-sheet decorated by four α-helices with two continuously connected grooves. A deep groove (groove-A) accommodates the AcCoA molecule. Interestingly, the acetyl end of AcCoA points toward an open space in a neighboring shallow groove (groove-S), which is occupied by extra electron density that potentially serves as a pseudosubstrate, suggesting that the groove-S may provide a substrate-binding site. Structure-based comparative analysis suggests that cjPseH utilizes a unique catalytic mechanism of acetylation that has not been observed in other glycosylation-associated acetyltransferases. Thus, our studies on cjPseH will provide valuable information for the design of new antibiotics to treat C. jejuni-induced gastroenteritis.
Organizational Affiliation:
Department of Systems Immunology, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Republic of Korea.