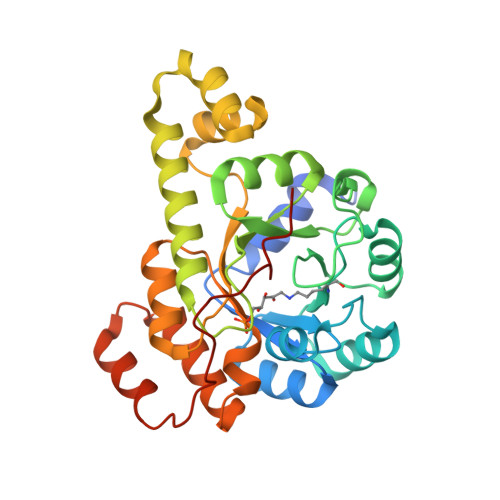
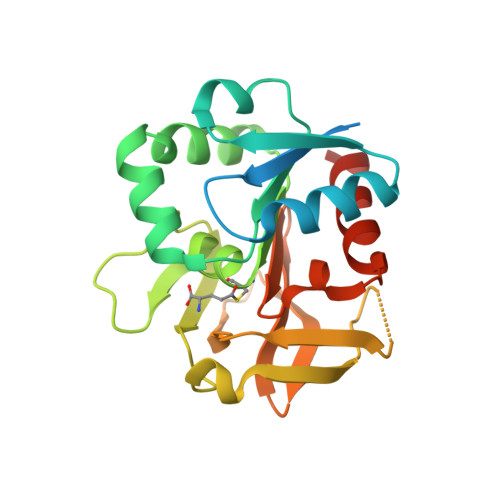
Crystal structures capture three states in the catalytic cycle of a pyridoxal phosphate (PLP) synthase.
Smith, A.M., Brown, W.C., Harms, E., Smith, J.L.(2015) J Biol Chem 290: 5226-5239
- PubMed: 25568319
- DOI: https://doi.org/10.1074/jbc.M114.626382
- Primary Citation of Related Structures:
4WXY, 4WXZ, 4WY0 - PubMed Abstract:
PLP synthase (PLPS) is a remarkable single-enzyme biosynthetic pathway that produces pyridoxal 5'-phosphate (PLP) from glutamine, ribose 5-phosphate, and glyceraldehyde 3-phosphate. The intact enzyme includes 12 synthase and 12 glutaminase subunits. PLP synthesis occurs in the synthase active site by a complicated mechanism involving at least two covalent intermediates at a catalytic lysine. The first intermediate forms with ribose 5-phosphate. The glutaminase subunit is a glutamine amidotransferase that hydrolyzes glutamine and channels ammonia to the synthase active site. Ammonia attack on the first covalent intermediate forms the second intermediate. Glyceraldehyde 3-phosphate reacts with the second intermediate to form PLP. To investigate the mechanism of the synthase subunit, crystal structures were obtained for three intermediate states of the Geobacillus stearothermophilus intact PLPS or its synthase subunit. The structures capture the synthase active site at three distinct steps in its complicated catalytic cycle, provide insights into the elusive mechanism, and illustrate the coordinated motions within the synthase subunit that separate the catalytic states. In the intact PLPS with a Michaelis-like intermediate in the glutaminase active site, the first covalent intermediate of the synthase is fully sequestered within the enzyme by the ordering of a generally disordered 20-residue C-terminal tail. Following addition of ammonia, the synthase active site opens and admits the Lys-149 side chain, which participates in formation of the second intermediate and PLP. Roles are identified for conserved Asp-24 in the formation of the first intermediate and for conserved Arg-147 in the conversion of the first to the second intermediate.
Organizational Affiliation:
From the Department of Biological Chemistry, Life Sciences Institute.