Agrochemical control of plant water use using engineered abscisic acid receptors.
Park, S.Y., Peterson, F.C., Mosquna, A., Yao, J., Volkman, B.F., Cutler, S.R.(2015) Nature 520: 545-548
- PubMed: 25652827
- DOI: https://doi.org/10.1038/nature14123
- Primary Citation of Related Structures:
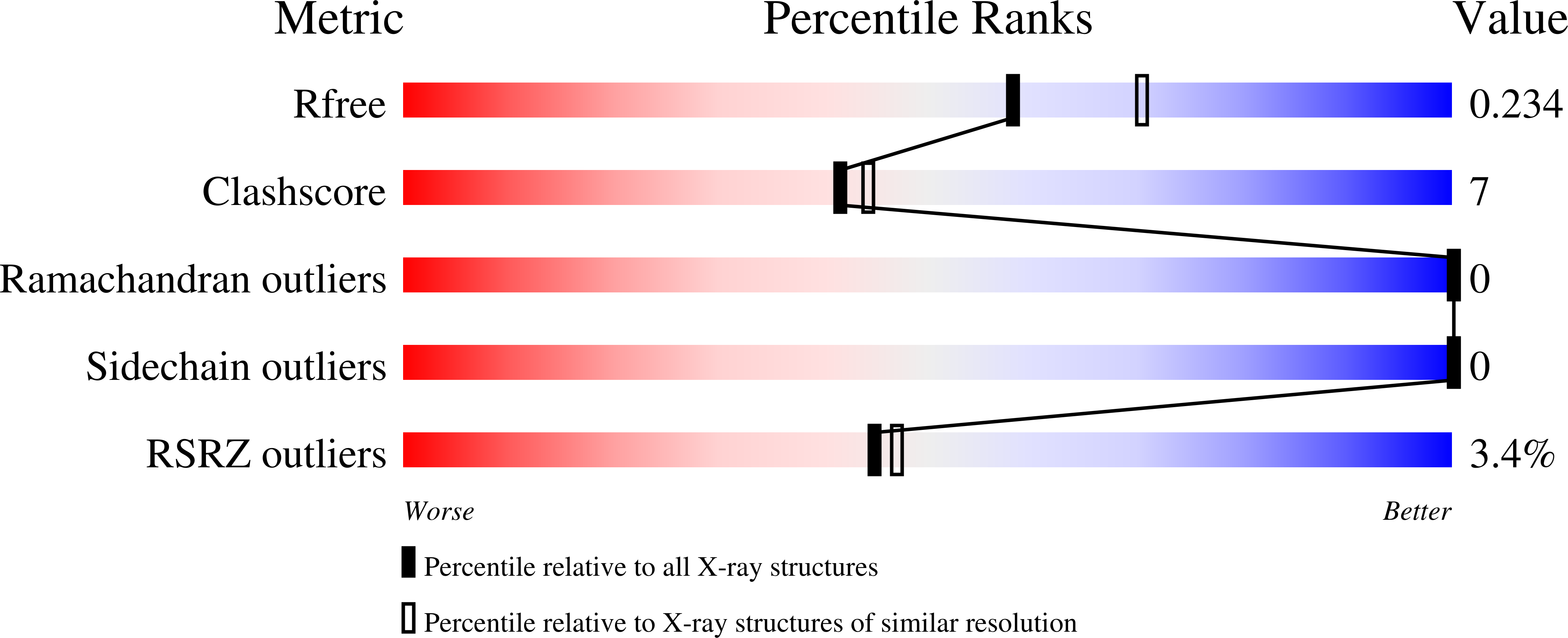
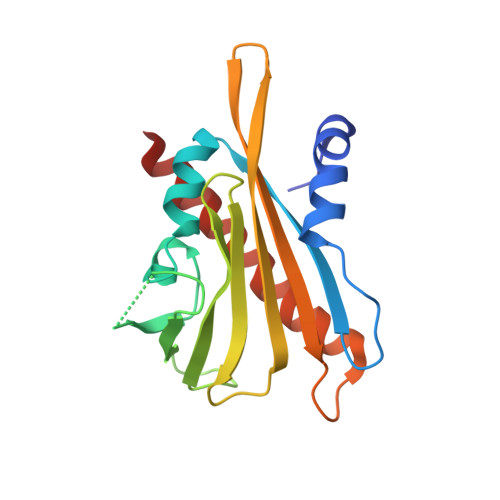
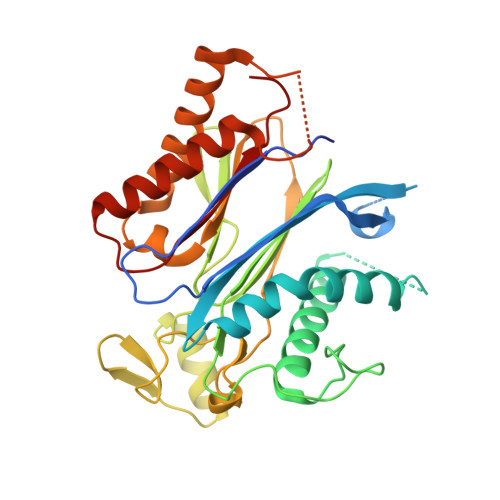
4WVO - PubMed Abstract:
Rising temperatures and lessening fresh water supplies are threatening agricultural productivity and have motivated efforts to improve plant water use and drought tolerance. During water deficit, plants produce elevated levels of abscisic acid (ABA), which improves water consumption and stress tolerance by controlling guard cell aperture and other protective responses. One attractive strategy for controlling water use is to develop compounds that activate ABA receptors, but agonists approved for use have yet to be developed. In principle, an engineered ABA receptor that can be activated by an existing agrochemical could achieve this goal. Here we describe a variant of the ABA receptor PYRABACTIN RESISTANCE 1 (PYR1) that possesses nanomolar sensitivity to the agrochemical mandipropamid and demonstrate its efficacy for controlling ABA responses and drought tolerance in transgenic plants. Furthermore, crystallographic studies provide a mechanistic basis for its activity and demonstrate the relative ease with which the PYR1 ligand-binding pocket can be altered to accommodate new ligands. Thus, we have successfully repurposed an agrochemical for a new application using receptor engineering. We anticipate that this strategy will be applied to other plant receptors and represents a new avenue for crop improvement.
Organizational Affiliation:
1] Center for Plant Cell Biology and Department of Botany and Plant Sciences, University of California, Riverside, California 92521, USA [2] Institute for Integrative Genome Biology, Riverside, California 92521, USA.