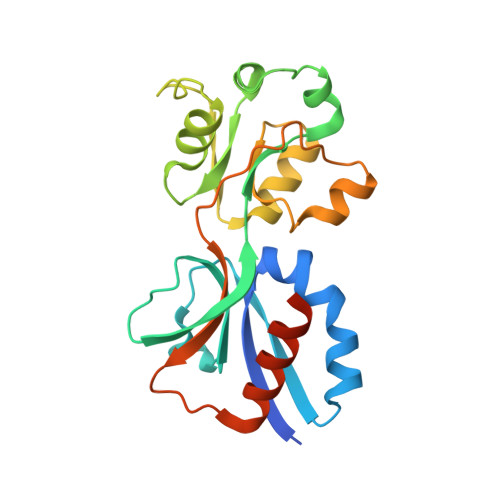
The beta-Lactamase Gene Regulator AmpR Is a Tetramer That Recognizes and Binds the d-Ala-d-Ala Motif of Its Repressor UDP-N-acetylmuramic Acid (MurNAc)-pentapeptide.
Vadlamani, G., Thomas, M.D., Patel, T.R., Donald, L.J., Reeve, T.M., Stetefeld, J., Standing, K.G., Vocadlo, D.J., Mark, B.L.(2015) J Biol Chem 290: 2630-2643
- PubMed: 25480792
- DOI: https://doi.org/10.1074/jbc.M114.618199
- Primary Citation of Related Structures:
4WKM - PubMed Abstract:
Inducible expression of chromosomal AmpC β-lactamase is a major cause of β-lactam antibiotic resistance in the Gram-negative bacteria Pseudomonas aeruginosa and Enterobacteriaceae. AmpC expression is induced by the LysR-type transcriptional regulator (LTTR) AmpR, which activates ampC expression in response to changes in peptidoglycan (PG) metabolite levels that occur during exposure to β-lactams. Under normal conditions, AmpR represses ampC transcription by binding the PG precursor UDP-N-acetylmuramic acid (MurNAc)-pentapeptide. When exposed to β-lactams, however, PG catabolites (1,6-anhydroMurNAc-peptides) accumulate in the cytosol, which have been proposed to competitively displace UDP-MurNAc-pentapeptide from AmpR and convert it into an activator of ampC transcription. Here we describe the molecular interactions between AmpR (from Citrobacter freundii), its DNA operator, and repressor UDP-MurNAc-pentapeptide. Non-denaturing mass spectrometry revealed AmpR to be a homotetramer that is stabilized by DNA containing the T-N11-A LTTR binding motif and revealed that it can bind four repressor molecules in an apparently stepwise manner. A crystal structure of the AmpR effector-binding domain bound to UDP-MurNAc-pentapeptide revealed that the terminal D-Ala-D-Ala motif of the repressor forms the primary contacts with the protein. This observation suggests that 1,6-anhydroMurNAc-pentapeptide may convert AmpR into an activator of ampC transcription more effectively than 1,6-anhydroMurNAc-tripeptide (which lacks the D-Ala-D-Ala motif). Finally, small angle x-ray scattering demonstrates that the AmpR·DNA complex adopts a flat conformation similar to the LTTR protein AphB and undergoes only a slight conformational change when binding UDP-MurNAc-pentapeptide. Modeling the AmpR·DNA tetramer bound to UDP-MurNAc-pentapeptide predicts that the UDP-MurNAc moiety of the repressor participates in modulating AmpR function.
Organizational Affiliation:
From the Departments of Microbiology.