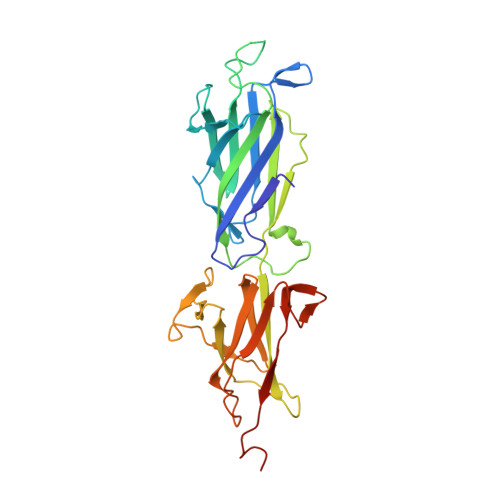
Structure and assembly of group B streptococcus pilus 2b backbone protein.
Cozzi, R., Malito, E., Lazzarin, M., Nuccitelli, A., Castagnetti, A., Bottomley, M.J., Margarit, I., Maione, D., Rinaudo, C.D.(2015) PLoS One 10: e0125875-e0125875
- PubMed: 25942637
- DOI: https://doi.org/10.1371/journal.pone.0125875
- Primary Citation of Related Structures:
4UZG - PubMed Abstract:
Group B Streptococcus (GBS) is a major cause of invasive disease in infants. Like other Gram-positive bacteria, GBS uses a sortase C-catalyzed transpeptidation mechanism to generate cell surface pili from backbone and ancillary pilin precursor substrates. The three pilus types identified in GBS contain structural subunits that are highly immunogenic and are promising candidates for the development of a broadly-protective vaccine. Here we report the X-ray crystal structure of the backbone protein of pilus 2b (BP-2b) at 1.06Å resolution. The structure reveals a classical IgG-like fold typical of the pilin subunits of other Gram-positive bacteria. The crystallized portion of the protein (residues 185-468) encompasses domains D2 and D3 that together confer high stability to the protein due to the presence of an internal isopeptide bond within each domain. The D2+D3 region, lacking the N-terminal D1 domain, was as potent as the entire protein in conferring protection against GBS challenge in a well-established mouse model. By site-directed mutagenesis and complementation studies in GBS knock-out strains we identified the residues and motives essential for assembly of the BP-2b monomers into high-molecular weight complexes, thus providing new insights into pilus 2b polymerization.
Organizational Affiliation:
Novartis Vaccines and Diagnostics, Siena, Italy.