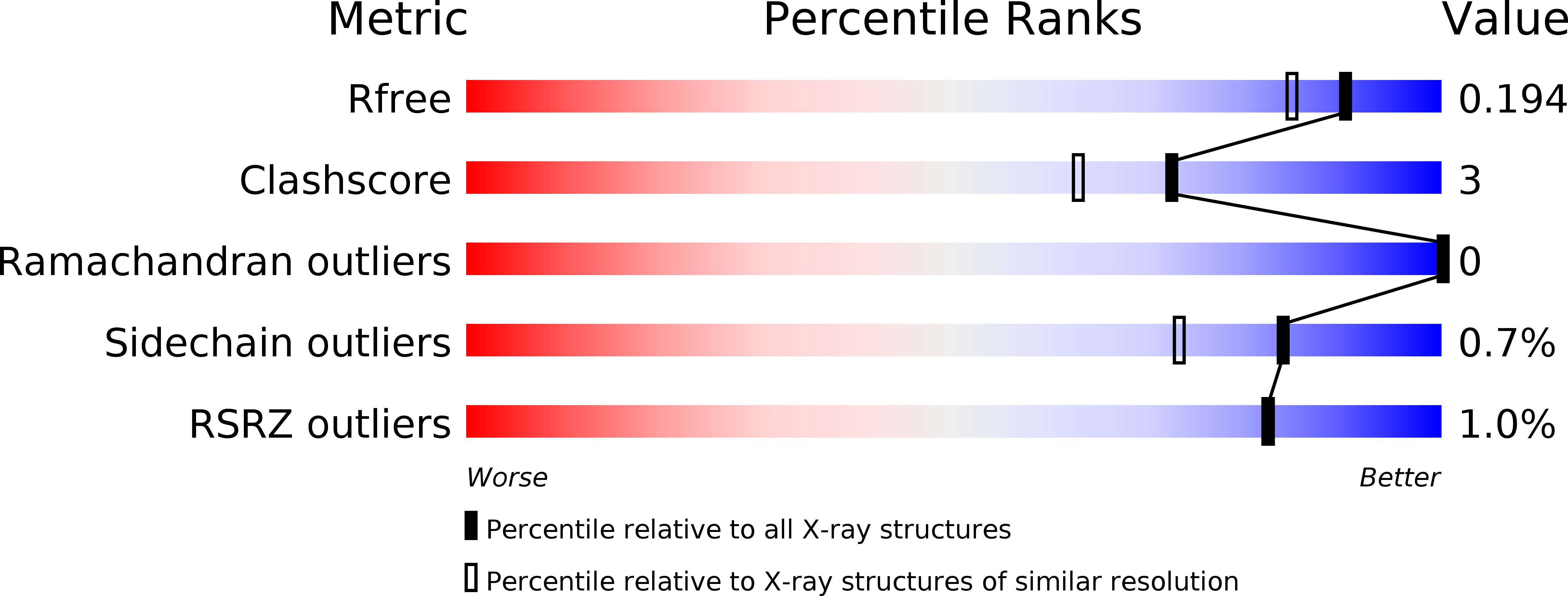
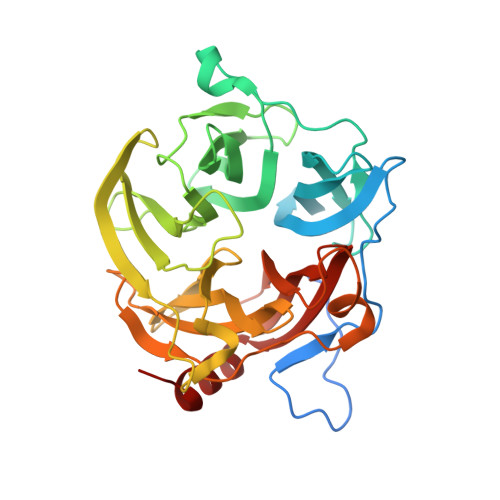
Structural Bases for N-Glycan Processing by Mannoside Phosphorylase.
Ladeveze, S., Cioci, G., Roblin, P., Mourey, L., Tranier, S., Potocki-Veronese, G.(2015) Acta Crystallogr D Biol Crystallogr 71: 1335
- PubMed: 26057673
- DOI: https://doi.org/10.1107/S1399004715006604
- Primary Citation of Related Structures:
4UDG, 4UDI, 4UDJ, 4UDK - PubMed Abstract:
The first crystal structure of Uhgb_MP, a β-1,4-mannopyranosyl-chitobiose phosphorylase belonging to the GH130 family which is involved in N-glycan degradation by human gut bacteria, was solved at 1.85 Å resolution in the apo form and in complex with mannose and N-acetylglucosamine. SAXS and crystal structure analysis revealed a hexameric structure, a specific feature of GH130 enzymes among other glycoside phosphorylases. Mapping of the -1 and +1 subsites in the presence of phosphate confirmed the conserved Asp104 as the general acid/base catalytic residue, which is in agreement with a single-step reaction mechanism involving Man O3 assistance for proton transfer. Analysis of this structure, the first to be solved for a member of the GH130_2 subfamily, revealed Met67, Phe203 and the Gly121-Pro125 loop as the main determinants of the specificity of Uhgb_MP and its homologues towards the N-glycan core oligosaccharides and mannan, and the molecular bases of the key role played by GH130 enzymes in the catabolism of dietary fibre and host glycans.
Organizational Affiliation:
Université de Toulouse; INSA, UPS, INP; LISBP, 135 Avenue de Rangueil, 31077 Toulouse, France.