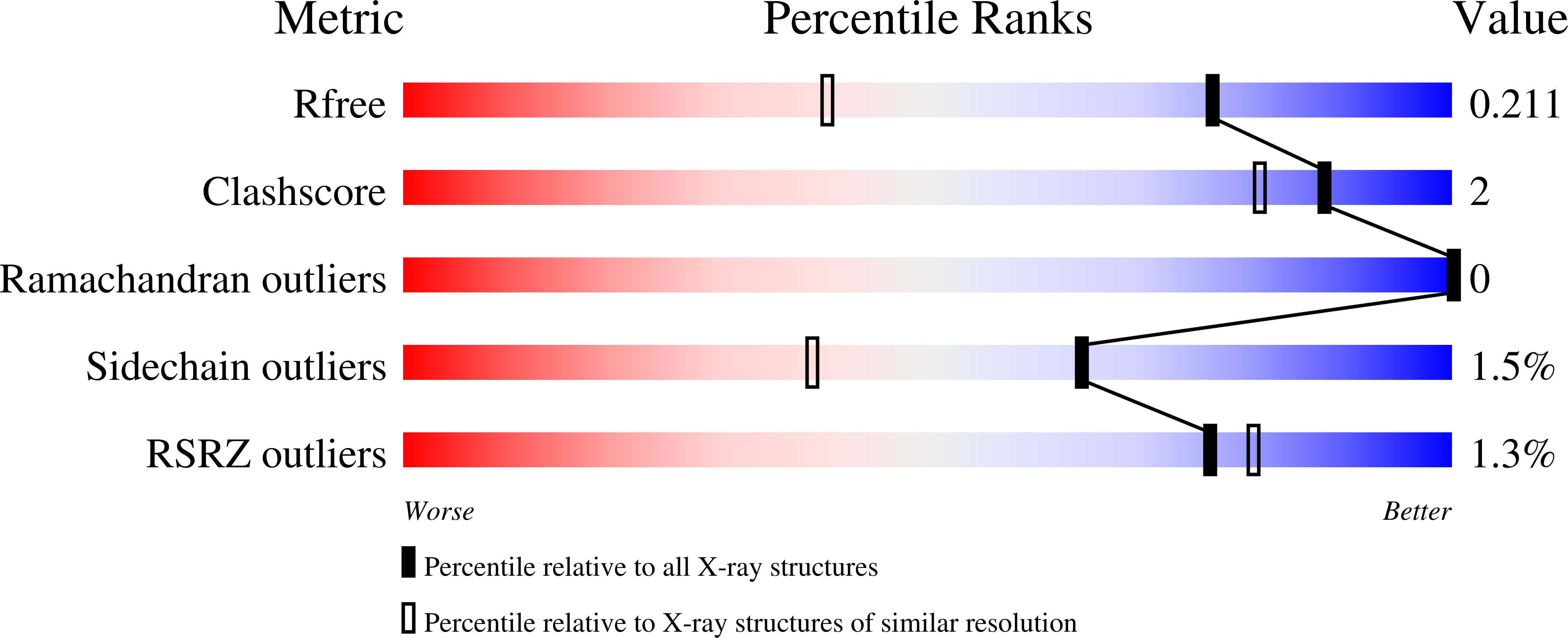
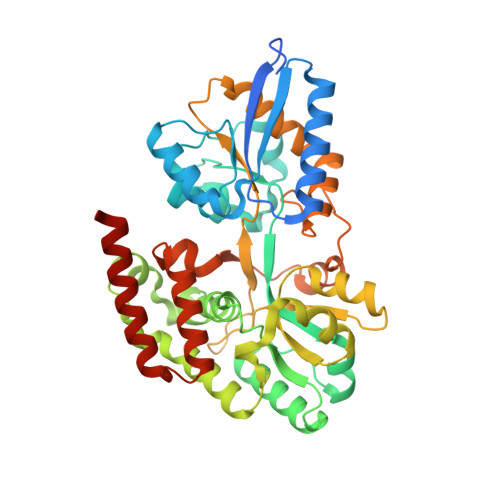
Crystal Structure of Transporter Msme from Bacillus Subtilis, Target Efi-510764
Patskovsky, Y., Toro, R., Bhosle, R., Al obaidi, N., Chamala, S., Scott glenn, A., Attonito, J.D., Chowdhury, S., Lafleur, J., Siedel, R.D., Hillerich, B., Love, J., Whalen, K.L., Gerlt, J.A., Almo, S.C., Enzyme Function Initiative (EFI)To be published.