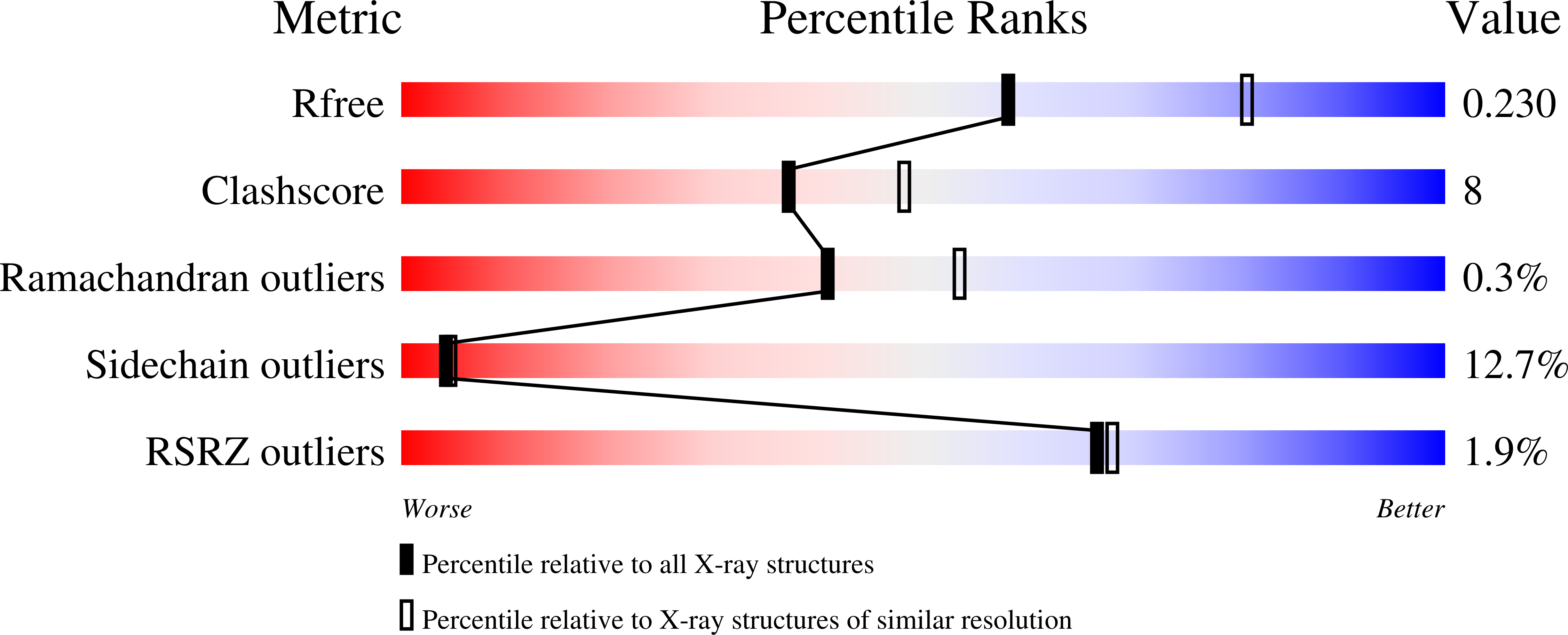
Binding-Pocket and Lid-Region Substitutions Render Human STING Sensitive to the Species-Specific Drug DMXAA.
Gao, P., Zillinger, T., Wang, W., Ascano, M., Dai, P., Hartmann, G., Tuschl, T., Deng, L., Barchet, W., Patel, D.J.(2014) Cell Rep 8: 1668-1676
- PubMed: 25199835
- DOI: https://doi.org/10.1016/j.celrep.2014.08.010
- Primary Citation of Related Structures:
4QXO, 4QXP, 4QXQ, 4QXR - PubMed Abstract:
The drug DMXAA (5,6-dimethylxanthenone-4-acetic acid) showed therapeutic promise against solid tumors in mouse models but subsequently failed in human clinical trials. DMXAA was later discovered to activate mouse, but not human, STING, an adaptor protein in the cyclic dinucleotide cGAMP-mediated signaling pathway, inducing type I interferon expression. To facilitate the development of compounds that target human STING, we combined structural, biophysical, and cellular assays to study mouse and human chimeric proteins and their interaction with DMXAA. We identified a single substitution (G230I) that enables a DMXAA-induced conformational transition of hSTING from an inactive "open" to an active "closed" state. We also identified a substitution within the binding pocket (Q266I) that cooperates with G230I and the previously identified S162A binding-pocket point substitution, rendering hSTING highly sensitive to DMXAA. These findings should facilitate the reciprocal engineering of DMXAA analogs that bind and stimulate wild-type hSTING and their exploitation for vaccine-adjuvant and anticancer drug development.
Organizational Affiliation:
Structural Biology Program, Memorial Sloan-Kettering Cancer Center, New York, NY 10065, USA.