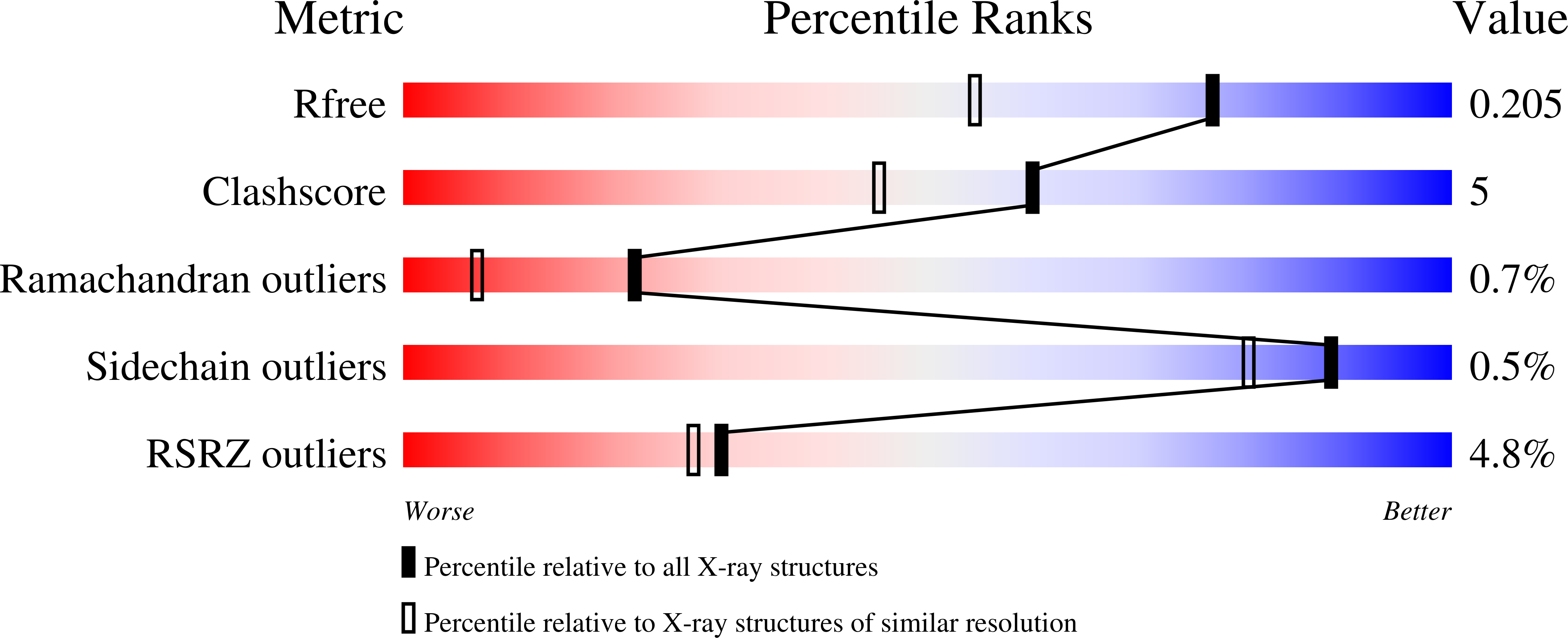
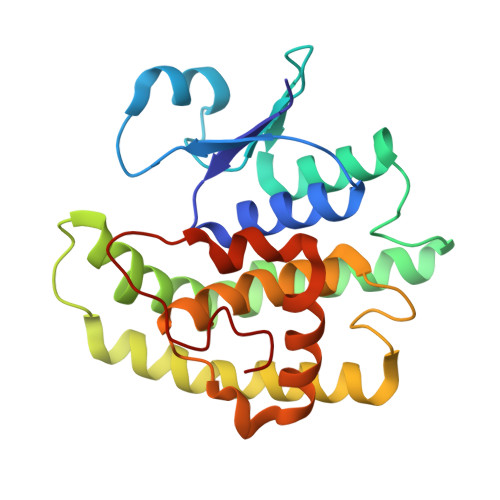
Crystal structure of a glutathione S-transferase from Rhodospirillum rubrum F11, Target EFI-507460
Kim, J., Toro, R., Bhosle, R., Al Obaidi, N.F., Morisco, L.L., Wasserman, S.R., Sojitra, S., Washington, E., Scott Glenn, A., Chowdhury, S., Evans, B., Stead, M., Hillerich, B., Love, J., Seidel, R.D., Imker, H.J., Attonito, J.D., Gerlt, J.A., Almo, S.C., Enzyme Function Initiative (EFI)To be published.