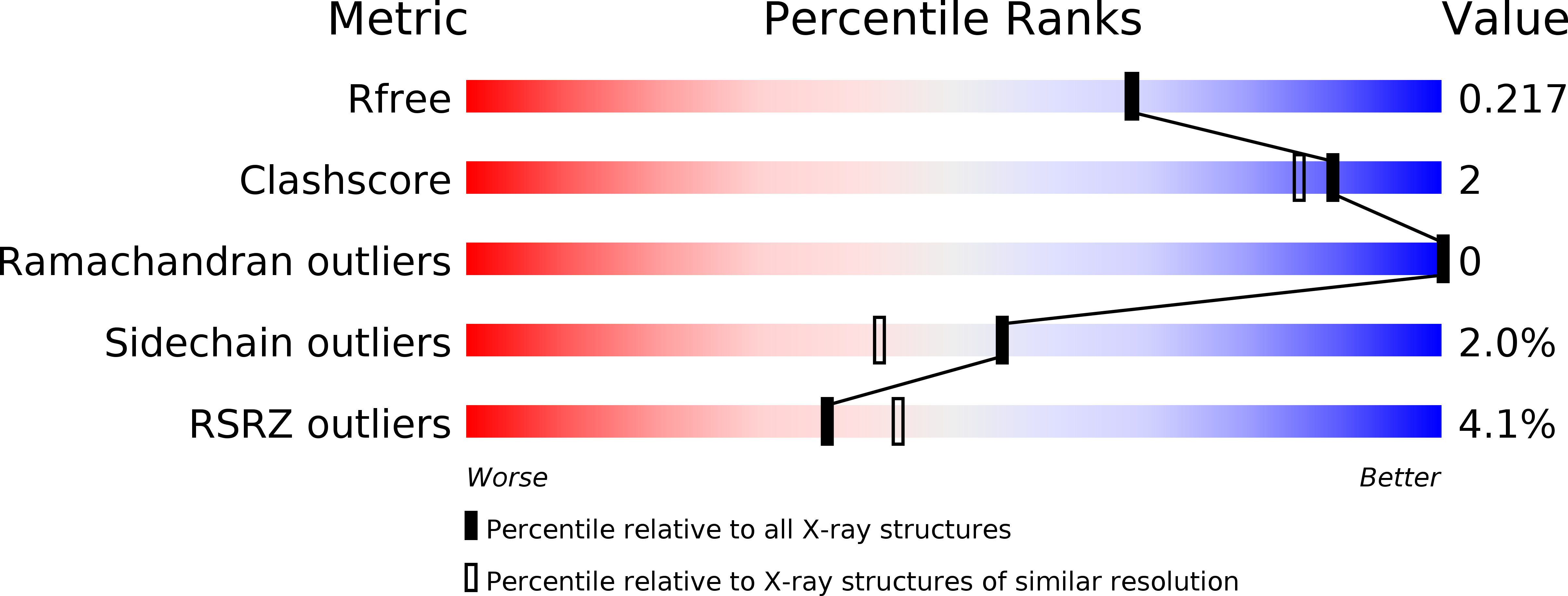
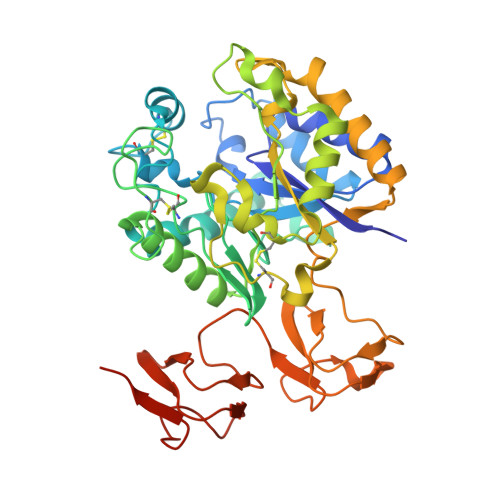
Structural basis of chitin oligosaccharide deacetylation.
Andres, E., Albesa-Jove, D., Biarnes, X., Moerschbacher, B.M., Guerin, M.E., Planas, A.(2014) Angew Chem Int Ed Engl 53: 6882-6887
- PubMed: 24810719
- DOI: https://doi.org/10.1002/anie.201400220
- Primary Citation of Related Structures:
4NY2, 4NYU, 4NYY, 4NZ1, 4NZ3, 4NZ4, 4OUI - PubMed Abstract:
Cell signaling and other biological activities of chitooligosaccharides (COSs) seem to be dependent not only on the degree of polymerization, but markedly on the specific de-N-acetylation pattern. Chitin de-N-acetylases (CDAs) catalyze the hydrolysis of the acetamido group in GlcNAc residues of chitin, chitosan, and COS. A major challenge is to understand how CDAs specifically define the distribution of GlcNAc and GlcNH2 moieties in the oligomeric chain. We report the crystal structure of the Vibrio cholerae CDA in four relevant states of its catalytic cycle. The two enzyme complexes with chitobiose and chitotriose represent the first 3D structures of a CDA with its natural substrates in a productive mode for catalysis, thereby unraveling an induced-fit mechanism with a significant conformational change of a loop closing the active site. We propose that the deacetylation pattern exhibited by different CDAs is governed by critical loops that shape and differentially block accessible subsites in the binding cleft of CE4 enzymes.
Organizational Affiliation:
Laboratory of Biochemistry, Institut Químic de Sarrià, Universitat Ramon Llull, Via Augusta 390, 08017 Barcelona (Spain).