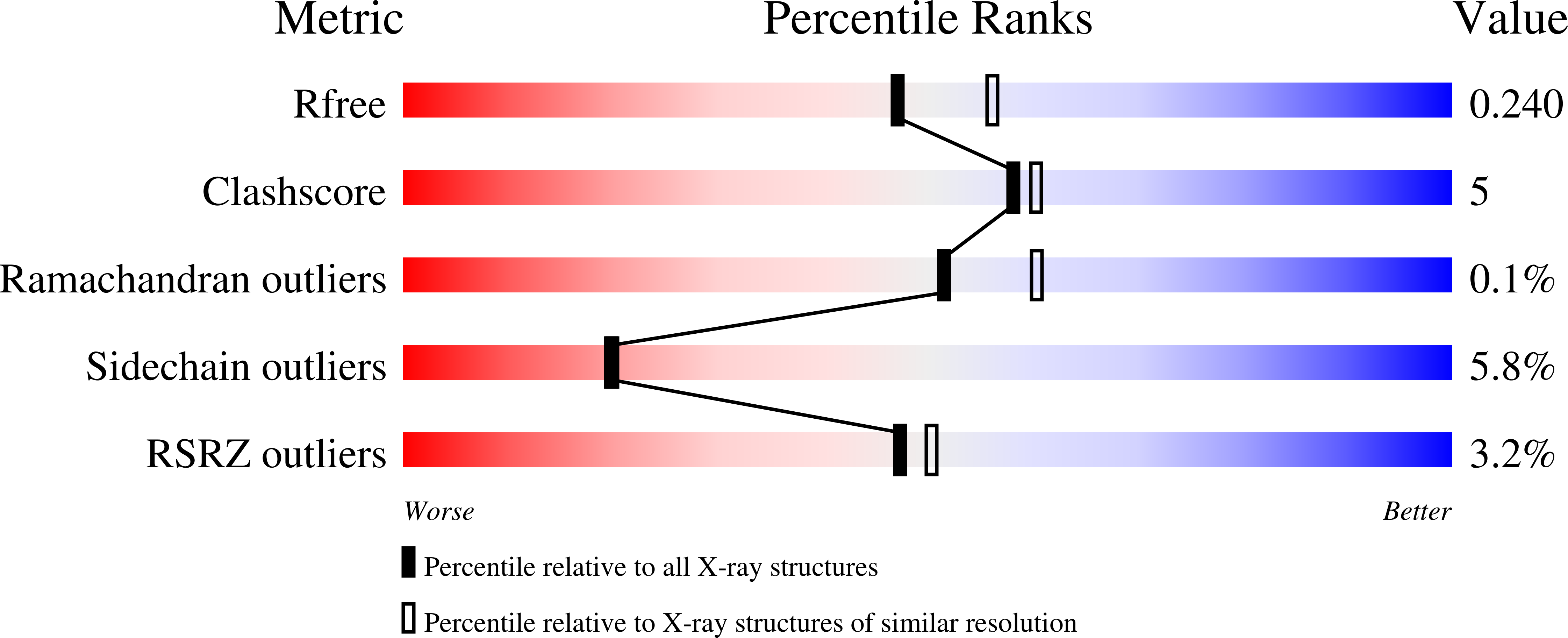
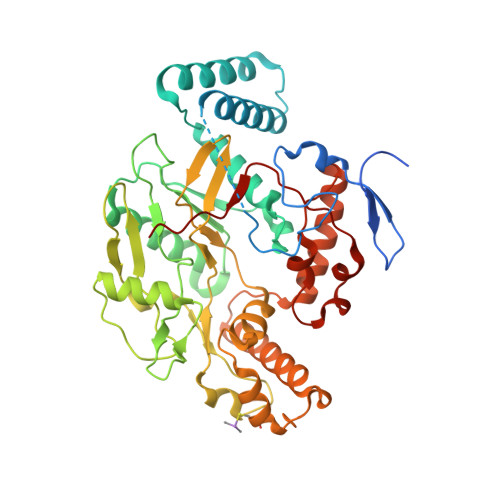
Structural and biological studies on bacterial nitric oxide synthase inhibitors.
Holden, J.K., Li, H., Jing, Q., Kang, S., Richo, J., Silverman, R.B., Poulos, T.L.(2013) Proc Natl Acad Sci U S A 110: 18127-18131
- PubMed: 24145412
- DOI: https://doi.org/10.1073/pnas.1314080110
- Primary Citation of Related Structures:
4LUW, 4LUX, 4LWA, 4LWB - PubMed Abstract:
Nitric oxide (NO) produced by bacterial NOS functions as a cytoprotective agent against oxidative stress in Staphylococcus aureus, Bacillus anthracis, and Bacillus subtilis. The screening of several NOS-selective inhibitors uncovered two inhibitors with potential antimicrobial properties. These two compounds impede the growth of B. subtilis under oxidative stress, and crystal structures show that each compound exhibits a unique binding mode. Both compounds serve as excellent leads for the future development of antimicrobials against bacterial NOS-containing bacteria.
Organizational Affiliation:
Departments of Molecular Biology and Biochemistry, Pharmaceutical Sciences, and Chemistry, University of California, Irvine, CA 92697-3900.